11.5: Theories of Sleep
- Page ID
- 110611
This page is a draft and under active development. Please forward any questions, comments, and/or feedback to the ASCCC OERI (oeri@asccc.org).
- Evaluate the functions of sleep in relation to the biological mechanisms underlying it and affected by it.
- Describe the circadian nature of sleep.
- Describe the homeostatic or recuperative theory of sleep, and other theories of why we sleep.
Overview
When you ask most people why they think they sleep – the answer is “so I can rest” or “because I am tired from the activities of the day.” However, the animals that work the hardest do not sleep the longest and even after pulling an all-nighter we don’t sleep for the entire following day to make up for the lack of sleep. There are two theories of sleep that work in conjunction to explain why we sleep – recuperative and circadian.
Research indicates that for someone to be able to fall asleep, two bodily processes must be properly synchronized. The first is the circadian rhythm, which has a 24-hour period and is governed by your body’s biological clock. The circadian rhythm controls the cyclical secretion of several hormones, including melatonin, that are involved in sleep. The second process is the recuperative function illustrated by the accumulation of hypnogenic substances in your body for 16 hours every day. These substances induce a desire to sleep that does not go away until you in fact get some sleep.
Thus you can fall asleep only when two conditions have been met: your body’s biological clock must have brought it into a hormonal balance conducive to sleep, and it must have been a good while since you last slept, so that your levels of hypnogenic (sleep-producing) substances have built up sufficiently. The following section describes these two theories in greater detail, as well as other functions of sleep.
Sleep Regulation and Circadian Rhythms: A Two-Process Model
Sleep is a dynamic process that adjusts to the body’s needs every day. What time you fall asleep, how long you sleep, and how well you sleep all result from the combined effects of two forces: the homeostatic debt and the phase of your circadian rhythm. Individuals of course differ as to what time they go to bed and how much sleep they need to function well, but on the whole, the characteristics of sleep can be regarded as the result of complex interactions between two processes.
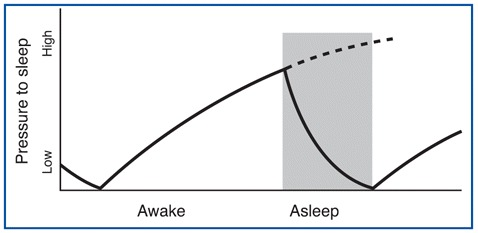
The first of these processes is called homeostatic debt (see below) or recuperative theory, which increases as a function of how long you have been awake and decreases as you sleep. The process is somewhat like the sand accumulating in one end of an hourglass and having to be emptied into the other after a certain time. See Figure \(\PageIndex{1}\) for a graph of how the pressure to sleep increases as a function of time spent awake, and then decreases as we sleep.
The second process that greatly influences the onset, duration, and quality of your sleep is the phase of your circadian rhythm. This phase is governed by your biological clock, whose rhythm is endogenous but is reset regularly by daylight. This clock therefore produces a cycle lasting about 24 hours during which the optimal times for falling asleep, dreaming, waking up, and doing work occur over the course of each day. The external cues or zeitgebers (German for "time-givers") - of which sunlight is the most important - entrain your endogenous (internal) body clock to fall in line with the earth's day.
Endogenous sleep rhythms can be depicted graphically. The figure shows a day-by-day representation of one individual's sleep/wake cycle. The black lines indicate periods of sleep, and the gray lines indicate periods of wakefulness. The upper portion of the figure (days 1 through 9) represents this individual's normal sleep/wake cycle. Under these conditions, the individual is exposed to regularly timed exposure to alternating daylight and darkness, which has entrained this person's sleep/wake cycling to a period of 24 hours.
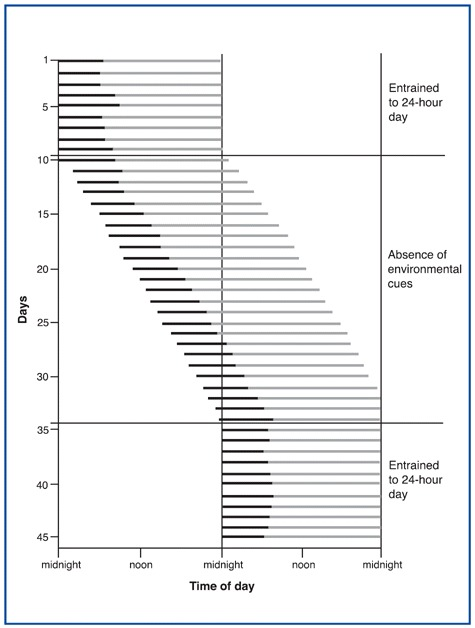
Thus your entire sleep-wake cycle operates as if your circadian oscillator made falling asleep easier at certain times of day, making you appreciably sleepier from 1:00 PM to 4:00 PM, and even sleepier from 2:00 AM to 5:00 AM. These patterns are confirmed by the statistics on workplace and highway accidents.
This two-process model - homeostatic and circadian - for regulating sleep can be diagrammed as a double pendulum.
Under normal circumstances, your periods of activity and rest are in phase with the alternation of day and night—your “circadian” pendulum and your “homeostatic” pendulum are in synch, you sleep well when you fall asleep, and you function well when you wake up.
But when this relationship is disturbed and the two pendulums are no longer in phase, then the quality both of your sleep and of your performance when awake deteriorate significantly. The peaks of activity for several circadian markers occur at inconvenient times in the sleep-wake cycle, which is the source of the problems caused by jet lag and by working night shifts.
In other words, the longer you stay awake, the greater the pressure you will feel to go to sleep. This process of homeostatic debt, or sleep debt, also explains why, if you stay up all night, then the next night, not only will you sleep longer, but your percentage of deep sleep will be higher.
Molecules that build up and make you sleep – Adenosine and Melatonin
As each day draws to a close, you feel the need to lie down and go to sleep. The onset of sleep, which seems like such a simple phenomenon from a behavioral perspective, is actually quite complex from a molecular one.
Adenosine and homeostasis/recuperation
It was in the early 1980s that scientists first discovered the chemical mechanism by which drinking coffee helps people to stay awake: caffeine, the psychoactive substance in coffee, prevents adenosine from binding to certain neurons in the brain. Both the caffeine in coffee and the theophylline in tea are examples of such adenosine antagonists and are well known for their stimulant effects.
Once this discovery was made, adenosine became a subject of interest for more and more neurobiologists who were doing sleep research. Numerous animal experiments eventually confirmed that adenosine definitely plays a role in the sleep/wake cycle. Some of the experimental findings that led to this conclusion: a) blocking the effects of adenosine made animals more alert; b) injecting animals with an adenosine agonist (enhancer) caused them to fall asleep; c) in certain parts of the brain, the concentration of adenosine normally increases naturally during the day and decreases at night, but if animals are forced to stay awake at night, this concentration keeps increasing.
These experiments thus showed that adenosine, along with other chemicals such as serotonin and melatonin, is one of the molecules whose concentration in the brain influences the onset of sleep.
Adenosine is produced by the degradation of adenosine triphosphate (ATP), the molecule that serves as the “energy currency” for the body’s various cellular functions. The amount of adenosine produced in the brain thus reflects the activity level of its neurons and glial cells. The brain’s intense activity during periods of wakefulness consumes large amounts of ATP and hence causes adenosine to accumulate.
Glycogen=ATP reserves or energy currency of the brain
Brain uses energy --> ATP degrades into adenosine
More brain energy used = more adenosine accumulates
More adenosine levels --> non-REM sleep triggered
Non-REM sleep = less active brain --> recovery/rebuild glycogen stores
The accumulation of adenosine during waking periods is thus associated with the depletion of the ATP reserves stored as glycogen in the brain. The increased adenosine levels trigger non-REM sleep, during which the brain is less active, thus placing it in a recovery phase that is absolutely essential—among other things, to let it rebuild its stores of glycogen.
But how exactly does adenosine exert this influence? During periods of wakefulness, neuronal activity increases the concentration of adenosine, which has an inhibitory effect on a great many neurons. Among these are the neurons of the hormonal systems that are the most active when we are awake: the norepinephrine, acetylcholine, and serotonin systems. Experiments have shown, for example, that when the levels of adenosine in the basal forebrain are raised artificially, the neurons in this structure that project axons throughout the cortex produce less acetylcholine. As a result, cortical activity slows, and the individual falls asleep.
The synchronized brain activity characteristic of non-REM sleep can then become established. But once non-REM sleep has continued for a while, the adenosine levels begin to decline. The systems responsible for wakefulness can then start becoming more active, causing the individual to awaken and the cycle to begin all over again. Thus we see that the sleep/wake cycle involves a highly efficient negative feedback loop.
Circadian rhythms
There are two important structures for the circadian cycle that could be considered our biological clocks.
The suprachiasmatic nuclei and the pineal gland and melatonin
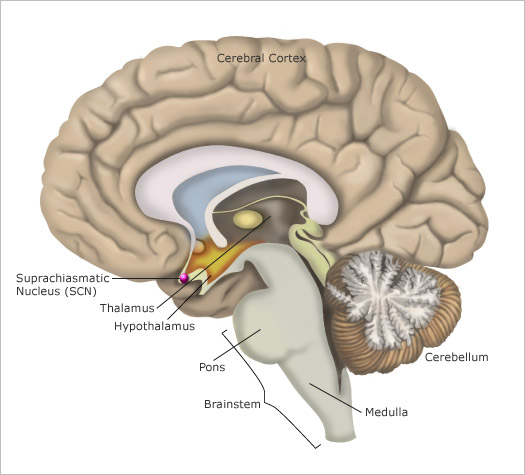
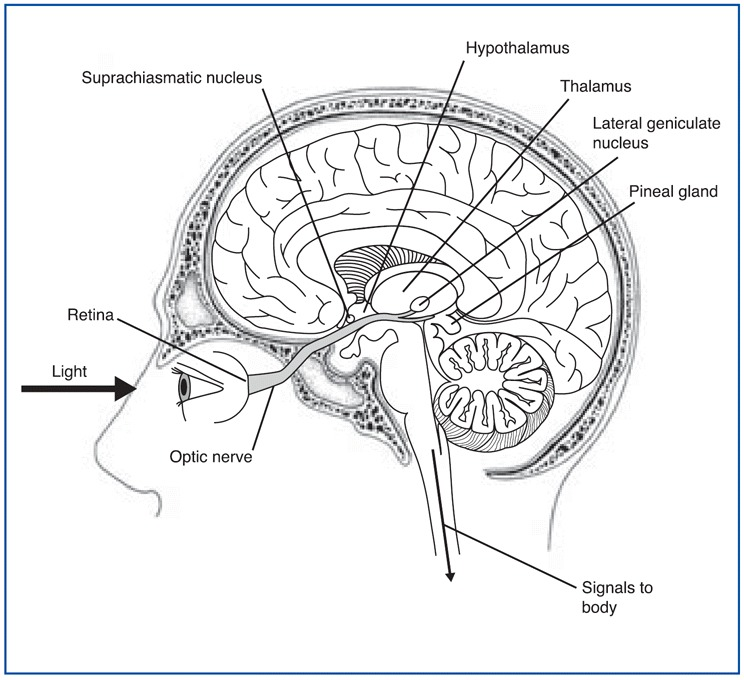
Most human bodily functions and behaviors are not “steady-state”. Instead, they fluctuate in 24-hour cycles, such as the sleeping and waking cycle and the cycles for body temperature, hunger, and the secretion of various hormones. The central clock that regulates all of these circadian cycles is located in two tiny structures in the brain, at the base of the left and right hypothalamus. Each of these structures is no larger than a pencil tip and contains several tens of thousands of neurons. These structures are called the suprachiasmatic nuclei (as mentioned earlier in section *) because they are located just above the optic chiasma, where the left and right optic nerves cross paths.
This strategic position enables the suprachiasmatic nuclei to receive projections from the optic nerve from special retinal ganglion cells that tell them about the intensity of the ambient light entering the eyes. The neurons of these nuclei use this information to resynchronize themselves with daylight every day, because like any clock, the human biological clock is not perfect and does need to be reset periodically. One interesting thing about the retinohypothalamic path is that it is separate from those for vision such that even blind people (and blind mole rats, a species that is otherwise blind) receive information from light to reset their biological clocks.
Despite this need to resynchronize with an external cue, it has been shown that the suprachiasmatic nuclei do in fact constitute a biological clock with its own independent rhythm. First, many experiments have shown that the fluctuations of the human circadian cycle persist even when individuals are cut off from the light of day. Second, in experiments where the suprachiasmatic nuclei were destroyed in animals such as hamsters, their cyclical behaviors, such as their sleep/wake cycles, become completely disorganized. And when suprachiasmatic nuclei were then transplanted from hamster fetuses into these animals, their biological rhythms returned, but with the properties of the donors.
These findings indicate that the mammalian biological clock mechanism is in fact not only endogenous, but also of genetic origin. Scientists have now even determined that these rhythms are the result of the cyclical activity of certain genes.
The circuit of light entering the eye, the signal going to the SCN and to the pineal gland is shown in the figure \(\PageIndex{4}\). Melanopsin in retinal ganglion cells in the eye respond to light (natural or artificially) and transmit signals to the SCN. Then light-induced activation of the SCN prevents the pineal gland from producing melatonin and; conversely, melatonin production and secretion is increased during the dark period.
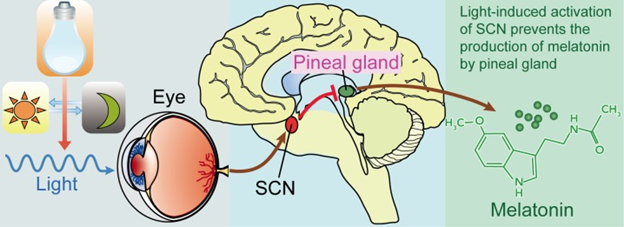
Pineal gland
Using these cyclical rhythms, the suprachiasmatic nuclei send signals along their output pathways–for example, to the pineal gland–to regulate the cycles of a number of physiological and behavioral functions. In birds, reptiles, and fish, this small gland located at the top of the brain is sensitive to light and co-ordinates some cyclical phenomena on its own. In mammals, however, though the pineal gland (mentioned earlier in section 12.2) does retain its ability to produce secretions cyclically (specifically, the hormone melatonin, at night), it does not constitute a clock on its own; instead, its cyclical synthesis of melatonin is controlled by timing signals that it receives from the SCN.
Each day, the pineal gland begins to produce melatonin (sometimes called the “sleep hormone”) as night falls. As the level of melatonin in the blood rises, body temperature falls slightly, and the individual feels sleepier and sleepier. The melatonin level remains high for just about 12 hours, then starts falling again in the early morning, as daylight (via the SCN) inhibits this gland’s activity.
The main neurotransmitter regulating the activity of the pineal gland is norepinephrine. When norepinephrine binds to its receptors, it triggers a cascade of second messengers, including cyclic AMP. This cyclic AMP contributes to the synthesis of melatonin. This melatonin is released into the bloodstream, through which it reaches every organ in the body. That is how it participates in the modulation of the circuits of the brainstem that ultimately control the sleep-wake cycle.
Output of SCN
The output pathways from each SCN (see Figure \(\PageIndex{4}\) for another picture of its location) consist of axons that innervate mainly the hypothalamus and nearby structures. Some of these axons also project to other parts of the forebrain, while others project to the midbrain.
Scientists do not yet know the details of how the central biological clock in the SCN regulates so many different human cyclical behaviors. But they do know that it uses the pineal gland to do so, and they have shown that destroying the SCN's output pathways also destroys the body’s circadian rhythms. Because GABA is the essential neurotransmitter for almost all of the SCN's neurons, one would expect an inhibitory effect on the neurons that they innervate. In addition to sending out messages along these axonal pathways, the SCN's neurons seem to secrete a neuropeptide called vasopressin in a cyclical pattern.
Yet another figure \(\PageIndex{5}\) shows the relative location of the different structures involved in the regulation of the circadian rhythms.
Scientists have now discovered that the neurotransmitter GABA excites the cells of the dorsal SCN but inhibits those of the ventral SCN. These opposing effects might influence the differing reaction times of these two sub-regions when someone travels across several time zones. This discovery thus opens new insights into the mechanisms behind the disturbing symptoms of jet lag.
Scientists removed neurons from the suprachiasmatic nuclei of rats and isolating these cells in a culture medium in vitro and found that feedback loops inside each of the cells discharge at frequencies varying in cycles lasting about 24 hours. But unlike suprachiasmatic nucleus cells in the brain, which synchronize their activity with the day/night cycle, suprachiasmatic nucleus cells in vitro do not. Like any other clock, the human body’s biological clock needs to be reset periodically. For that to happen, every cell in this clock must resynchronize itself daily with external cues that tell it when the day begins and ends. These external cues, also known as Zeitgebers (German for “time givers”), include the ambient temperature, the consumption of meals, ambient noise, and the body’s activity level. But the strongest of these cues is undoubtedly the overall intensity of the ambient light.
Resetting the body's internal clocks - Zeitgebers
Light-sensitive ganglion cells
Thanks to various tagging methods, scientists now know that a certain sub-population of the ganglion cells in the human retina contain a photosensitive pigment and project their axons directly into the suprachiasmatic nuclei as well as into other brain structures that are concerned with the intensity of ambient light.
These light-sensitive ganglion cells have large receptive fields, because of their long, widely dispersed dendrites. In these cells, accurate reception of information on shape, orientation, and movement is sacrificed to general sensitivity. These cells clearly constitute another light-sensitive system that runs parallel to the visual system but is dedicated to detecting light intensity rather than to forming images.
Molecular clockwork
Many human functions, such as alertness, body temperature, and the secretion of certain hormones, work better if they are adjusted according to whether it is day or night. As you might therefore expect, a mechanism has evolved within the human body to co-ordinate its major functions with the time of day.
The human biological clock is extremely regular, though not exactly 24 hours: it is accurate to within 1%. But just like a watch that is never absolutely accurate on its own and needs to be reset occasionally, this biological clock needs a mechanism to prevent tiny errors from accumulating in each cell. Also, it needs to synchronize itself with external signs that tell it when each new day begins. The growing intensity of the natural light is the first sign of daybreak, and special photopigments in the retina detect this change in light intensity and transmit this information to the human biological clock.
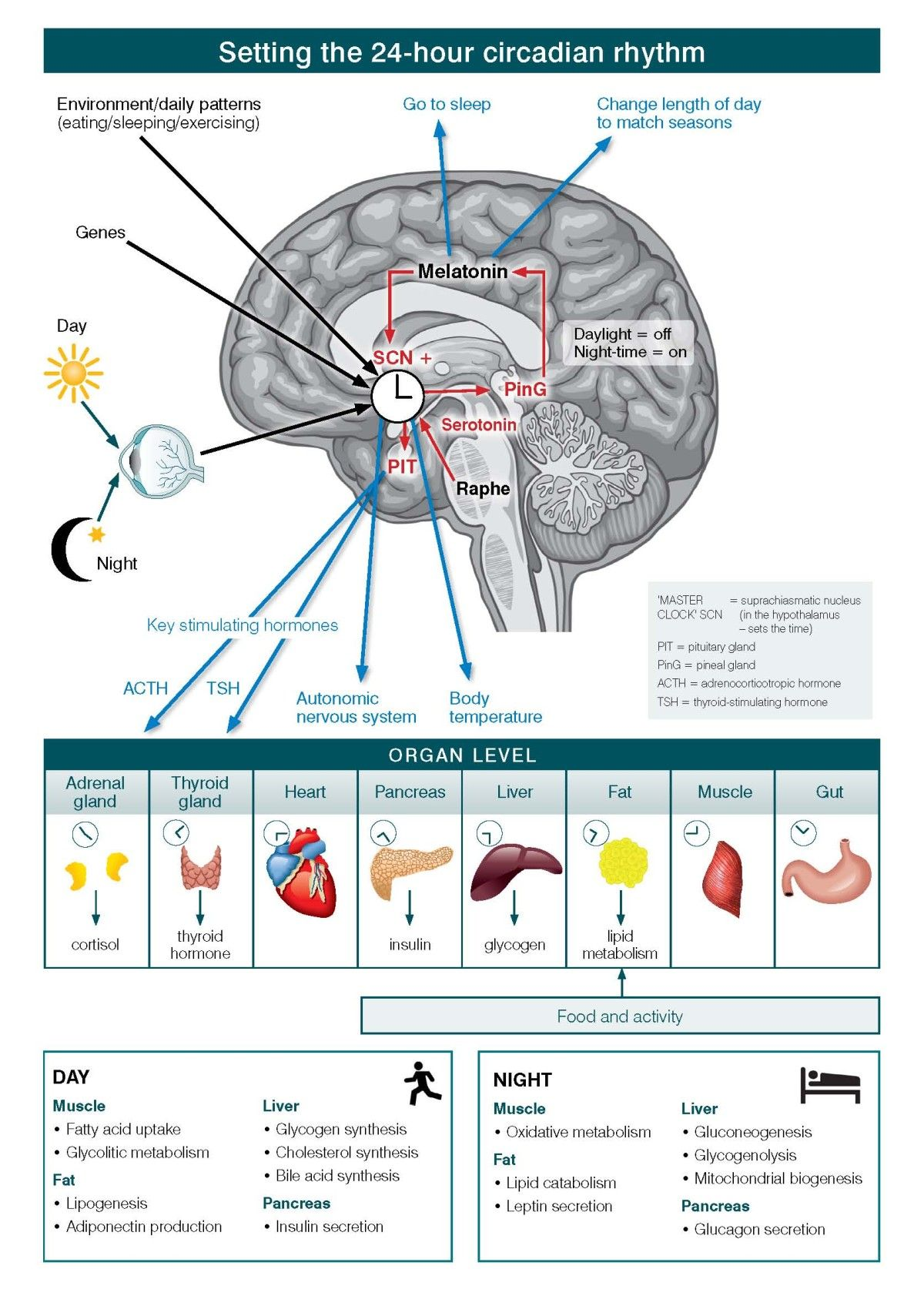
In Figure \(\PageIndex{7}\), the levels of organization of information and operation of the circadian systems are shown. Circadian systems need to be considered in relation to three differing levels of organization of information and operation. First is the way in which the physical environment communicates (or ‘Inputs’) key information, particularly related to differentiation of night from day, to the internal ‘master’ clock (located in the brain’s suprachiasmatic nucleus (SCN)). Second are the ‘Intrinsic’ brain factors, consisting of the master clock and its linked regulatory systems (notably secretion of melatonin from the pineal gland). These contribute to sleep onset, sleep architecture, sleep-wake cycles and other central nervous system (CNS)-dependent behavioral changes. Third is the way in which the circadian system coordinates all other hormonal, metabolic, immune, thermoregulatory, autonomic nervous and other physiological processes to optimize the relationships between behavior and body functions (that is, the ‘Outputs’).
At the cellular level, almost all individual cells and, hence, organ systems have their own intrinsic clocks. As these cellular (for example, fibroblasts, fat cells, muscles) and organ-based (for example, liver, pancreas, gut) clocks run to intrinsically different period lengths, the differing physiological systems need to be aligned in coherent patterns. Fundamentally, the master circadian clock permits the organism to align key behavioral and intrinsic physiological rhythms optimally to the external 24-hour light–dark cycle.
In the figure, the black arrows from eye (and sunlight), genes, and environment/daily patterns (eating/sleeping/exercising) to the SCN indicate input received by the master clock. The red arrows from the raphe nuclei and pineal gland (via melatonin) to the SCN, and from the SCN to the pituitary indicate intra-CNS modulation of the signals. The blue arrows from melatonin to sleep regulation signals, from the SCN to ANS activity and temperature regulation, and pituitary to hormone regulation (ACTH and TSH) indicate output from the brain controlling other organ systems in the body.
The table at the end illustrates how muscle, liver, fat and pancreas function changes depending on time of day or night. During the day, muscle uptake fatty acid, and increase glycolitic metabolism. For fats, there is lipogenesis and adiponectin production. In the liver, there is glycogen, cholesterol and bile acid synthesis. In the pancreas there is insulin secretion. At night, muscles engage in oxidative metabolism. Fats have lipid catabolism and leptin secretion. The liver engages in gluconeogenesis, glycogenolysis, and mitochondrial biogenesis. The pancreas secrete glucagon.
Other theories of why we sleep
As mentioned in section * there are many functions of sleep including memory consolidation, energy conservation, brain development and discharge of emotions. Further, the importance of sleep is apparent in the multiple negative ramifications of sleep deprivation.
Summary
Under normal circumstances, there are two main mechanisms by which our sleep is regulated - homeostatic or recuperative and circadian or rhythmic. Adenosine appears to be the main chemical underlying the homeostatic/recuperative functions of sleep. Indeed most of us are aware of how we feel tired after long periods of wakefulness. Melatonin and the free running rhythms of the SCN seem to underlie the circadian/rhythmic aspects of sleep. Jet lag, shift work, and the overall disruption of people's body clocks during the COVID pandemic provide testimony to how this theory is also an important basis of sleep. Certainly it is clear that humans need sleep for a variety of reasons and that when we are sleep deprived, there are many mental and physical health consequences.
References
Attributions
- Molecules that build up and make you sleep - intermediate by Bruno Dubuc under a Copyleft license.
- Molecules that build up and make you sleep - beginner by Bruno Dubuc under a Copyleft license.
- The suprachiasmatic nuclei and the pineal gland - intermediate by Bruno Dubuc under a Copyleft license.
- Light-sensitive ganglion cells - advanced by Bruno Dubuc under a Copyleft license.
- Cyclic behavior of animals by CK-12: Biology Concepts licensed CC BY-NC 4.0
- Our Molecular Clockwork - intermediate by Bruno Dubuc under a Copyleft license.
- Our Molecular Clockwork - beginner by Bruno Dubuc under a Copyleft license.
- Sleep and Dreaming by Lumen Boundless Psychology licensed CC BY-SA
- Sagittal image of brain licensed CC BY-SA
- Light, suprachiasmatic nuclei (SCN) and the pinealmelatonin ciruit.jpg by Zhiqiang Ma, Yang Yang, Chongxi Fan, Jing Han, Dongjin Wang, Shouyin Di, Wei Hu, Dong Liu, Xiaofei Li, Russel J. Reiter, and Xiaolong Yan, licensed CC BY 4.0, via Wikimedia Commons
- Tool Module: Sleep Regulation and Circadian Rhythms by Bruno Dubuc under a Copyleft license.
- The master circadian clock in the human brain.jpg by Ian B Hickie, Sharon L Naismith, Rébecca Robillard, Elizabeth M Scott, and Daniel F Hermens, licensed CC BY 3.0, via Wikimedia Commons


