13.6: Sex Differences and Evolutionary Interpretations
- Page ID
- 136061
This page is a draft and under active development. Please forward any questions, comments, and/or feedback to the ASCCC OERI (oeri@asccc.org).
- Explain sex differences that have been reported in the brain, sensory systems and cognitive functions, and the limitations of these findings
- Describe how hormones may influence sex differences in behavior, such as childhood toy preferences and aggressive behaviors
- Identify the core premises of sexual strategies theory
Overview
After noting that the dichotomy does not truly reflect the variations seen in both biological sex and gender identity, this section discusses sex differences that have been reported between human males and females. Sex differences in the brain, sex differences in sensory systems and cognition, and sex differences in behavior are covered, with the last topic including a review of hormonal influences on sex differences in behavior followed by discussions of childhood toy preferences and aggressive behaviors. Finally, evolutionary interpretations of reproductive behavior is addressed, including sexual strategies theory and error management theory (as it relates to reproductive behaviors).
Sex Differences
Preliminary Note
Earlier sections have emphasized the lack of a strict dichotomy between males and females, both in terms of biological sex and gender identity. Nonetheless, most research splits people into two categories- most commonly based on their biological sex assigned at birth- and often also assumes that the biological sex reported correlates with gender identity. While this is useful to a degree, hopefully future research will take a more nuanced approach to all three aspects of an individual's "sexual" self- sex, gender, and sexual orientation! You will note below that when we compare men and women on psychological traits and characteristics, there is actually only a slight difference (typically no more than 5%) between the two, and only at the group level. Think of it this way- adult men in the U.S. are about 9% taller than adult women, on average (McDowell et al., 2008), and about 15% larger in body mass (Larsen, 2003). Yet everyone can think of examples of men who are shorter and/or smaller than many women, and women who are taller and/or larger than many men- and this physical difference is at least twice the size of any of the differences found in psychological domains!
Also, as you will learn in the section on sex differences in the brain, while differences between male and female brains have been reported at a group level, there is no way to reliably classify an individual brain as either male or female, due to both the large overlap between males and females and the fact that each brain has its own unique mosaic of both "male" and "female" traits in differing (and unpredictable) ways.
Introduction to Sex Differences
Hens and roosters are different (Figure \(\PageIndex{1}\)). Cows and bulls are different. Men and women are different. Even girls and boys are different. Humans, like many animals, are sexually dimorphic (di, “two”; morph, “type”) in the size and shape of their bodies, their physiology, and for our purposes, their behavior.

Interestingly, the physical differences in size and appearance (for many individuals, we can readily determine biological sex from visible traits) between human males and females is related to some of the differences we see in human reproductive behaviors. In the animal kingdom, many animals whose mating strategy is to form monogamous pairs have virtually indistinguishable males and females, whereas polygamous species (where one male typically mates with many females, and some males completely miss out on mating) are conspicuously different, often in both physical features and in size. While apparent in some bird species, such as the obviously different polygamous rooster and hen mentioned above (versus monogamous swans, with nearly identical males and females), these differences are particularly evident in (non-human) primate species (and many other mammals). Examples in primate species include the similarly-appearing monogamous gibbons (Jaffe, 2019, page 210-211), versus the polygamous gorillas (Jaffe, 2019, page 209), where adult males are much larger than females and gain the characteristic "silverback" coloring as they mature (Figure \(\PageIndex{2}\)). A difference of around ten percent- precisely where the human species falls on this size-difference continuum- is the dividing line between "monogamous" and "polygamous"; in other words, species that have less than ten-percent difference in physical appearance and size between males and females are usually monogamous and species that have greater than ten-percent difference tend to be polygamous. For humans, "...the sexes differ more in human beings than in monogamous mammals, but much less than in extremely polygamous mammals..." (Daly & Wilson, 1996, page 13). Thus, it is not really surprising, from an evolutionary standpoint, that some humans adopt a monogamous strategy for mating and reproduction, and others follow a polygamous strategy.
_(2854166549).jpg?revision=1&size=bestfit&width=450&height=300)
.jpg?revision=1&size=bestfit&width=451&height=300)
Returning to human behavior, the behavior of boys and girls differs in many ways. Girls generally excel in verbal abilities relative to boys; boys are nearly twice as likely as girls to suffer from dyslexia (reading difficulties) and stuttering, and nearly four times more likely to suffer from autism. Boys are generally better than girls at tasks that require visuospatial abilities. Girls engage in nurturing behaviors more frequently than boys. More than 90% of all anorexia nervosa cases involve young women. Young men are twice as likely as young women to suffer from schizophrenia. Boys are much more physically aggressive and generally engage in more rough-and-tumble play than girls (Berenbaum, Martin, Hanish, Briggs, & Fabes, 2008). Many behavioral sex differences, such as the difference in physical aggressiveness, persist throughout adulthood. For example, there are many more men than women serving prison sentences for violent behavior. Below, this section addresses sex differences in the brain, in sensory systems and cognition, and in behavior, where sex differences in toy preferences and the previously mentioned aggressive behavior are discussed in greater detail.
Sex Differences in the Brain
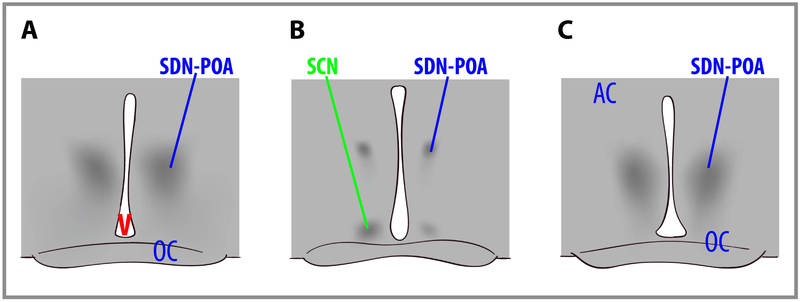
Sex differences in overall human brain size have been reported for years (although the true significance of this finding is debatable). More recently, sex differences in specific brain structures have been discovered. For example, in rats, the sexually dimorphic nucleus (SDN-POA) of the preoptic area of the hypothalamus is much larger in males than females. This is a result of the organizing effects of gonadal steroid hormones upon brain and behavior, which is relatively constrained to the early stages of development. In rats, exposure to testosterone (which is converted to estradiol) or estradiol causes masculinization of the brain. (The same mechanism of a "female" hormone masculinizing the fetal brain is not present in primates, and thus presumably not in humans. Alpha-fetoprotein binds to estradiol in female rats to prevent masculinization of their brains.) Figure \(\PageIndex{3}\) depicts cross-sections through the brains of rats that show a male (A, left), a female (B, center), and a female treated with testosterone as a newborn (C, right). Note that the SDN-POA (the dark cell bodies) of the male are substantially larger than those of the untreated female but are equal in size to those of the testosterone-treated female. The extent that these sex differences in brain structure account for sex differences in behavior remain unspecified in mammals.
In an area similar to the rat's SDN-POA, Simon LeVay (1991) reported a comparable difference in the human hypothalamus- INAH-3, the third interstitial nucleus of the anterior hypothalamus. Specifically, heterosexual men had INAH-3 nuclei that were more than twice as large (by volume) as homosexual men, whose INAH-3 nuclei volumes were similar to those of women. Although LeVay was able to replicate the result, attempts by another researcher lead to only a trend-level difference between heterosexual and homosexual men in the volume of INAH-3 (Byne 2000 & 2001, as cited by Bailey et al., 2016). Regardless of the true status of the possible differences in the human male's INAH-3 nucleus (it could be intermediate between the two researchers' findings, for example), "it would be unlikely that the INAH-3 size would be a key factor regulating sexual orientation. This is because there would be too many exceptions—homosexual men with a large INAH-3 and heterosexual men with a small INAH-3—to believe that INAH-3 size is crucial" (Bailey et al., 2016, page 72). The INAH-3 is a tiny structure- about the size of a grain of sand- and studying it requires post-mortem tissue. As such, it is unlikely that it will be studied further in the near future (Bailey et al., 2016).
For larger brain features, which can be studied in living people using imaging techniques, many brain differences between adult men and women have been reported, both in cortical regions and subcortical structures. An example of this includes magnetic resonance imaging (MRI) data suggesting that women have larger volumes of frontal and medial paralimbic cortices and men have larger volumes in the frontomedial cortex, amygdala and hypothalamus (Goldstein et al., 2001). However, closer scrutiny sometimes renders these sex differences unimportant. For example, the corpus callosum was widely believed to differ between males and females- the purported increased size in women was touted to result in greater connectivity between the right and left hemispheres of the brain- but has been found to not differ at all when men and women were matched for brain size (Luders et al., 2014). Another study underlining the importance of taking brain size into consideration was a twin study that found that a weak correlation between sex differences in the brain and behavioral sex differences turned out to be mainly driven by brain size. The authors warned that caution should therefore be used when inferring causality (van Eijk et al., 2021).
Additionally, Joel et al. (2015) conducted an extensive study of over 1400 MRIs of human brains and found that when taking the entire brain into account (and not just looking at one specific area versus another, where sex differences have been reported at a group level), it is impossible to categorize brains into male and female forms. Instead, there was extensive overlap of “male” and “female” features in human brains and it was very rare to have internal consistency (only “male” or only “female” features) in a specific brain. Thus, while we can often categorize biological sex by looking at anatomical structures- there are often distinct differences between male and female bodies, and a given body usually has only male or only female structures- we cannot determine if a specific brain came from a male or female body, nor can we predict by knowing the sex/gender of a particular individual what form their brain has taken. The authors state that “each brain is a unique mosaic of features, some of which may be more common in females compared with males, others may be more common in males compared with females, and still others may be common in both males and females” (Joel et al., 2015, page 15472). Additionally, their findings support the notion that masculinization and feminization of brain areas are two separate processes that progress independently, allowing variations of differentiation in a “male” or “female” pattern to occur within the same brain.
Sex Differences in Sensory Systems and Cognition
Sex differences in a number of sensory and cognitive functions have also been reported. Females are generally more sensitive to auditory information, whereas males are more sensitive to visual information. Females are also typically more sensitive than males to taste and olfactory input. Women display less lateralization of cognitive functions than men. On average, females generally excel in verbal, perceptual, and fine motor skills, whereas males outperform females on quantitative and visuospatial tasks, including map reading and direction finding. Interestingly, women with Turner syndrome (whose ovaries are not functional) often have impaired spatial memory.
Although reliable sex differences can be documented, these differences in ability are slight. It is important to note that there is more variation within each sex than between the sexes for most cognitive abilities (Figure \(\PageIndex{4}\)).
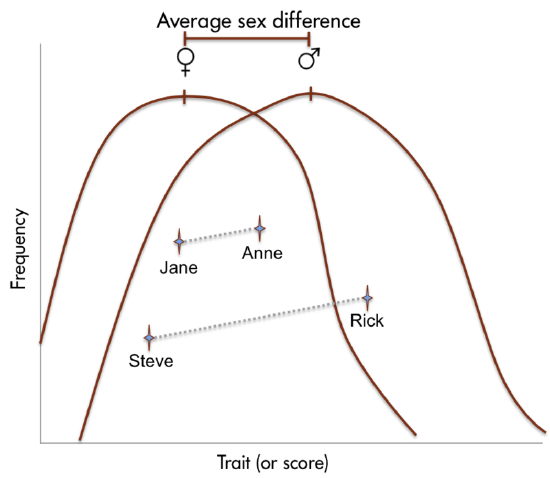
To emphasize the importance of recognizing the vast variation of personality traits and behavioral expressions found within all humans, regardless of their sex/gender, consider the following study results. Joel et al. (2015) conducted an analysis of the personality traits, attitudes, interests, and behaviors of more than 5,500 individuals. Their findings mirrored their MRI findings (great overlap between males and females and little internal consistency within the same individual). They report: “In accordance with the brain data, our analyses of gender-related data revealed extensive overlap between females and males in personality traits, attitudes, interests, and behaviors. Moreover, we found that substantial variability of gender characteristics is highly prevalent, whereas internal consistency is extremely rare, even for highly gender-stereotyped activities... Thus, most humans possess a mosaic of personality traits, attitudes, interests and behaviors, some more common in males compared with females, others more common in females compared with males, and still others common in both males and females” (Joel et al., 2015, page 15472). It is reasonable to conclude that overall, human males and females are more similar than they are different, both with regards to brain features and psychological traits.
Sex Differences in Behavior
Review of Hormonal Influences on Sex Differences in Behavior
The hormonal differences between men and women may account for adult sex differences that develop during puberty, but what accounts for behavioral sex differences among children prior to puberty and activation of their gonads? As discussed in section 13.2, the presence (or absence) of androgens determine whether the fetus develops a male or female body form. Androgens from the fetal testes steer the development of the body, central nervous system, and subsequent behavior in a male direction. In contrast, fetal ovaries do not secrete high concentrations of hormones, so the body, central nervous system, and later, behavior, follow a female pathway.
As discussed in section 13.1, gonadal steroid hormones have organizational (or programming) effects upon brain and behavior (Phoenix, Goy, Gerall, & Young, 1959). These organizing effects are relatively constrained to the early stages of development. In rats, early steroid hormone treatment causes relatively irreversible and permanent masculinization of later adult behavior (mating and aggressive). In contrast, activational effects of hormones (provided in adulthood to influence behaviors) are temporary and reversible. Thus, typical male behavior requires exposure to androgens during gestation (in humans) or immediately after birth (in rats) to somewhat masculinize the brain and also requires androgens during or after puberty to activate these neural circuits. Typical female behavior requires a lack of exposure to androgens early in life which leads to feminization of the brain and also requires estrogens to activate these neural circuits in adulthood. But this simple dichotomy, which works well with animals with very distinct sexual dimorphism in behavior, has many caveats when applied to people.
Childhood Toy Preferences
If you walk through any major toy store, then you will likely observe a couple of aisles filled with pink boxes and the complete absence of pink packaging of toys in adjacent aisles. Remarkably, you will also see a strong self-segregation of boys and girls in these aisles. It is rare to see boys in the “pink” aisles and vice versa. The toy manufacturers are often accused of making toys that are gender biased, but it seems more likely that boys and girls enjoy playing with specific types and colors of toys. Indeed, toy manufacturers would immediately double their sales if they could sell toys to both sexes. Boys generally prefer toys such as trucks and balls and girls generally prefer toys such as dolls. Although it is doubtful that there are genes that encode preferences for toy cars and trucks on the Y chromosome, it is possible that hormones might shape the development of a child’s brain to prefer certain types of toys or styles of play behavior. It is also reasonable to believe that children learn which types of toys and which styles of play are appropriate to their gender (Figure \(\PageIndex{5}\)). How can we separate the contribution of learning from underlying physiological mechanisms to understand sex differences in human behaviors? To untangle these issues, animal models are often used. Unlike the situation in humans, where sex differences are usually only a matter of degree (often slight), in some animals, members of only one sex may display a particular behavior. For example, often only male songbirds sing. Studies of such strongly sex-biased behaviors are particularly valuable for understanding the interaction among behavior, hormones, and the nervous system.


A study of vervet monkeys calls into question the primacy of learning in the establishment of toy preferences (Alexander & Hines, 2002). Female vervet monkeys preferred girl-typical toys, such as dolls or cooking pots, whereas male vervet monkeys preferred boy-typical toys, such as cars or balls. There were no sex differences in preference for gender-neutral toys, such as picture books or stuffed animals. Presumably, monkeys have no prior concept of “boy” or “girl” toys. Young rhesus monkeys also show similar toy preferences.
What then underlies the sex difference in toy preference? It is possible that certain attributes of toys (or objects) appeal to either boys or girls. Toys that appeal to boys or male vervet or rhesus monkeys, in this case, a ball or toy car, are objects that can be moved actively through space, toys that can be incorporated into active, rough and tumble play. The appeal of toys that girls or female vervet monkeys prefer appears to be based on color. Pink and red (the colors of the doll and pot) may provoke attention to infants.
Society may reinforce such stereotypical responses to gender-typical toys. The sex differences in toy preferences emerge by 12 or 24 months of age and seem fixed by 36 months of age, but are sex differences in toy preference present during the first year of life? It is difficult to ask pre-verbal infants what they prefer, but in studies where the investigators examined the amount of time that babies looked at different toys, eye-tracking data indicate that infants as young as three months showed sex differences in toy preferences; girls preferred dolls, whereas boys preferred trucks. Another result that suggests, but does not prove, that hormones are involved in toy preferences is the observation that girls diagnosed with congenital adrenal hyperplasia (CAH), whose adrenal glands produce varying amounts of androgens early in life, played with masculine toys more often than girls without CAH. Further, a dose-response relationship between the extent of the disorder (i.e., degree of fetal androgen exposure) and degree of masculinization of play behavior was observed. Are the sex differences in toy preferences or play activity, for example, the inevitable consequences of the differential hormonal environments of boys and girls, or are these differences imposed by cultural practices and beliefs? Are these differences the result of receiving gender-specific toys from an early age, or are these differences some combination of hormonal and cultural factors? Again, these are difficult questions to unravel in people.
Aggressive Behaviors
Note that in this section "aggressive behavior" is referring specifically to the expression of physical aggression (arguably the only type of aggression that can be studied in animals as well as humans). When aggression is defined more broadly to include relational aggression (such as non-physical bullying, gossiping, and humiliation), human males and females show much more similar overall levels of aggression (Swearer Napolitano, 2008).
The possibility for aggressive behavior exists whenever the interests of two or more individuals are in conflict (Nelson, 2006). Conflicts are most likely to arise over limited resources such as territories, food, and mates. A social interaction decides which animal gains access to the contested resource. In many cases, a submissive posture or gesture on the part of one animal avoids the necessity of actual combat over a resource. Animals may also participate in threat displays or ritualized combat in which dominance is determined but no physical damage is inflicted.
There is overwhelming circumstantial evidence that androgens mediate aggressive behavior across many species. First, seasonal variations in blood plasma concentrations of testosterone and seasonal variations in aggression coincide. For instance, the incidence of aggressive behavior peaks for male deer in autumn, when they are secreting high levels of testosterone. Second, aggressive behaviors increase at the time of puberty, when the testes become active and blood concentrations of androgens rise. Juvenile deer do not participate in the fighting during the mating season. Third, in any given species, males are generally more aggressive than females. This is certainly true of deer; relative to stags, female deer rarely display aggressive behavior, and their rare aggressive acts are qualitatively different from the aggressive behavior of males. Finally, castration (removal of the testes) typically reduces aggression in males, and testosterone replacement therapy restores aggression to pre-castration levels. There are some interesting exceptions to these general observations that are outside the scope of this section.
As mentioned, males are generally more aggressive than females. Certainly, human males are much more physically aggressive than females. Many more men than women are convicted of violent crimes in North America. The sex differences in human aggressiveness appear very early. At every age throughout the school years, many more boys than girls initiate physical assaults. Almost everyone will acknowledge the existence of this sex difference, but assigning a cause to behavioral sex differences in humans always elicits much debate. It is possible that boys are more aggressive than girls because androgens promote aggressive behavior and boys have higher blood concentrations of androgens than girls. It is possible that boys and girls differ in their aggressiveness because the brains of boys are exposed to androgens prenatally and the “wiring” of their brains is thus organized in a way that facilitates the expression of aggression (Figure \(\PageIndex{6}\)). It is also possible that boys are encouraged and girls are discouraged by family, peers, or others from acting in an aggressive manner. These three hypotheses are not mutually exclusive, but it is extremely difficult to discriminate among them to account for sex differences in human aggressiveness.

What kinds of studies would be necessary to assess these hypotheses? It is usually difficult to separate out the influences of environment and physiology on the development of behavior in humans. For example, boys and girls differ in their rough-and-tumble play at a very young age, which suggests an early physiological influence on aggression. This pattern of more physical play behavior is seen in a number of other species including nonhuman primates, rats, and dogs. Is the difference in the frequency of rough-and-tumble play between boys and girls due to biological factors associated with being male or female, or is it due to cultural expectations and learning? Parents interact with their male and female offspring differently; they usually play more roughly with male infants than with females, which suggests that the sex difference in aggressiveness is partially learned. This difference in parental interaction style is evident by the first week of life. If there is a combination of biological and cultural influences mediating the frequency of rough-and-tumble play, then what proportion of the variation between the sexes is due to biological factors and what proportion is due to social influences? Importantly, is it appropriate to talk about “normal” sex differences when these traits virtually always arrange themselves along a continuum rather than in discrete categories? Because of these complexities in the factors influencing human behavior, the study of hormonal effects on sex-differentiated behavior has been pursued in nonhuman animals, where it is easier to hold environmental influences relatively constant.
With the appropriate animal model, we can address the questions posed above: Is the sex difference in aggression due to higher adult blood concentrations of androgens in males than in females, or are males more aggressive than females because their brains are organized differently by perinatal hormones? Are males usually more aggressive than females because of an interaction of early and current blood androgen concentrations? If male mice are castrated prior to their sixth day of life, then treated with testosterone in adulthood, they show low levels of aggression. Similarly, female mice ovariectomized prior to their sixth day but given androgens in adulthood do not express male-like levels of aggression. Treatment of perinatally gonadectomized males or females with testosterone prior to their sixth day life and also in adulthood results in a level of aggression similar to that observed in typical male mice. Thus, in mice, the proclivity for males to act more aggressively than females is organized perinatally by androgens but also requires the presence of androgens after puberty in order to be fully expressed. In other words, aggression in male mice is both organized and activated by androgens. Testosterone exposure in adulthood without prior organization of the brain by steroid hormones does not evoke typical male levels of aggression. The hormonal control of aggressive behavior in mice is thus similar to the hormonal mediation of heterosexual male mating behavior in other rodent species. Aggressive behavior is similarly both organized and activated by androgens in many species, including rats, hamsters, voles, dogs, and possibly some primate species.
Evolutionary Interpretations of Reproductive Behavior

Evolutionary psychology connects evolutionary principles with modern psychology and focuses primarily on psychological adaptations: changes in the way we think in order to improve our survival. (See Chapter 3 for more information on evolution.) This section describes one of the major evolutionary psychological theories that is relevant to reproductive behaviors- sexual strategies theory- which describes the psychology of human mating strategies and the ways in which women and men differ in those strategies. Additionally, an example from another evolutionary psychological theory- error management theory- that is relevant to human mating is provided.
Sexual Strategies Theory
Sexual strategies theory is based on sexual selection theory- the evolution of characteristics due to mating advantage rather than survival advantage, such as traits that make an individual more attractive to the opposite sex, and competition between members of the same sex. It proposes that humans have evolved a list of different mating strategies, both short-term and long-term, that vary depending on culture, social context, parental influence, and personal mate value (desirability in the “mating market”). Initially the focus was on the differences between men and women in mating preferences and strategies (Buss & Schmitt, 1993), starting by looking at the minimum parental investment needed to produce a child. For women, even the minimum investment is significant: after becoming pregnant, they have to carry that child for nine months inside of them (Figure \(\PageIndex{7}\)). For men, on the other hand, the minimum investment to produce the same child is considerably smaller—simply the act of sex.

These differences in parental investment have an enormous impact on sexual strategies. For a woman, the risks associated with making a poor mating choice is high. She might get pregnant by a man who will not help to support her and her children, or who might have poor-quality genes. And because the stakes are higher for a woman, wise mating decisions for her are much more valuable. While this discussion is not intended in any way to endorse "bad behavior", for men, biologically speaking, the need to focus on making wise mating decisions isn’t as important. That is, unlike women, men 1) don’t biologically have the child growing inside of them for nine months, and 2) do not have as high a cultural expectation to raise the child. This logic leads to a powerful set of predictions: In short-term mating, women will likely be choosier than men (because the costs of getting pregnant are so high), while men, on average, will likely engage in more casual sexual activities (because this cost is greatly lessened). Due to this, men will sometimes deceive women about their long-term intentions for the benefit of short-term sex, and men are more likely than women to lower their mating standards for short-term mating situations.
An extensive body of empirical evidence supports these and related predictions (Buss & Schmitt, 2011). Men express a desire for a larger number of sex partners than women do. They let less time elapse before seeking sex. They are more willing to consent to sex with strangers and are less likely to require emotional involvement with their sex partners. They have more frequent sexual fantasies and fantasize about a larger variety of sex partners. They are more likely to regret missed sexual opportunities. And they lower their standards in short-term mating, showing a willingness to mate with a larger variety of women as long as the costs and risks are low.
However, it is also important to note that in situations where both the man and woman are interested in long-term mating, both sexes tend to invest substantially in the relationship and in their children. In these cases, the theory predicts that both sexes will be extremely choosy when pursuing a long-term mating strategy. Much empirical research supports this prediction, as well. In fact, the qualities women and men generally look for when choosing long-term mates are very similar: both want mates who are intelligent, kind, understanding, healthy, dependable, honest, loyal, loving, and adaptable.
Nonetheless, women and men do differ in their preferences for a few key qualities in long-term mating, because of somewhat distinct adaptive problems. Modern women have inherited the evolutionary trait to desire mates who possess resources, have qualities linked with acquiring resources (e.g., ambition, wealth, industriousness), and are willing to share those resources with them. On the other hand, men more strongly desire youth and health in women, as both are cues to fertility. These male and female differences are universal in humans. They were first documented in 37 different cultures, from Australia to Zambia (Buss, 1989), and have been replicated by dozens of researchers in dozens of additional cultures (for summaries, see Buss, 2012).
As we know, though, just because we have these mating preferences (e.g., men with resources; fertile women), people don't always get what they want. There are countless other factors which influence who people ultimately select as their mate. For example, the sex ratio (the percentage of men to women in the mating pool), cultural practices (such as arranged marriages, which inhibit individuals’ freedom to act on their preferred mating strategies), the strategies of others (e.g., if everyone else is pursuing short-term sex, it’s more difficult to pursue a long-term mating strategy), and many others all influence who we select as our mates.
Sexual strategies theory—anchored in sexual selection theory— predicts specific similarities and differences in men and women’s mating preferences and strategies. Whether we seek short-term or long-term relationships, many personality, social, cultural, and ecological factors will all influence who our partners will be.
Error Management Theory
How we think, make decisions, and evaluate uncertain situations (error management theory) has also been used to predict adaptive biases in the domain of mating. Consider something as simple as a smile. In one case, a smile from a potential mate could be a sign of sexual or romantic interest. On the other hand, it may just signal friendliness. Because of the costs to men of missing out on chances for reproduction, error management theory predicts that men have a sexual overperception bias: they often misread sexual interest from a woman, when really it’s just a friendly smile or touch. In the mating domain, the sexual overperception bias is one of the best-documented phenomena. It’s been shown in studies in which men and women rated the sexual interest between people in photographs and videotaped interactions. As well, it’s been shown in the laboratory with participants engaging in actual “speed dating,” where the men interpret sexual interest from the women more often than the women actually intended it (Perilloux, Easton, & Buss, 2012). In short, error management theory predicts that men, more than women, will over-infer sexual interest based on minimal cues, and empirical research confirms this adaptive mating bias.
Summary
Humans, like many animals, are sexually dimorphic in the size and shape of their bodies, their physiology, and for our purposes, their behavior. Girls generally excel in verbal abilities, whereas boys generally have better visuospatial abilities. Boys are more likely to suffer from dyslexia, stuttering, autism, and schizophrenia, while most anorexia nervosa cases involve young women. Girls engage in nurturing behaviors more frequently, and boys generally engage in more rough-and-tumble play. One behavioral sex difference that persists into adulthood is the higher levels of physical aggression exhibited by boys and men.
In rats, the sexually dimorphic nucleus of the preoptic area in the hypothalamus (SDN-POA) is much larger in males than females. A female rat treated with testosterone as a newborn develops an SDN-POA similar in size to a typical male rat. In humans, a similar brain area, the third interstitial nucleus of the anterior hypothalamus (INAH-3), was found to be larger in heterosexual men than homosexual men (whose INAH-3 was similar in size to those of women). However, replication outside of the initial lab did not reach statistical significance. Brain imaging studies have reported various differences in cortical regions and subcortical structures, but closer scrutiny sometimes renders these sex differences unimportant, and caution should be used when inferring causality. A study of over 1400 MRIs of human brains found that when taking the entire brain into account, it is impossible to categorize brains into male and female forms. There was extensive overlap of “male” and “female” features in human brains and it was very rare to have internal consistency in a specific brain. These findings also support the notion that masculinization and feminization of brain areas are two separate processes that progress independently, allowing for considerable variation across individuals.
Sex differences in a number of sensory and cognitive functions have also been reported, but these differences in ability are slight. Furthermore, there is more variation within each sex than between the sexes for most cognitive abilities. An analysis of personality traits, attitudes, interests, and behaviors of more than 5,500 individuals mirrored the MRI findings (great overlap between males and females and little internal consistency within the same individual). It is reasonable to conclude that overall, human males and females are more similar than they are different, both with regards to brain features and psychological traits.
Boys generally prefer toys such as trucks and balls and girls generally prefer toys such as dolls. It is possible that hormones shape the development of a child’s brain to prefer certain types of toys or styles of play behavior, but children also learn which types of toys and which styles of play are appropriate to their gender. Studies of vervet monkeys and rhesus monkeys find that females prefer girl-typical toys, and males prefer boy-typical toys, with no sex differences in preference for gender-neutral toys. While society likely reinforces stereotypical responses to gender-typical toys in human children, sex differences in toy preferences emerge by 12 or 24 months of age and seem fixed by 36 months of age. Studies using eye-tracking indicate that infants as young as three months show stereotypical sex differences in toy preferences. Additionally, in a dose-response relationship, girls diagnosed with congenital adrenal hyperplasia (CAH) played with masculine toys more often than girls without CAH, suggesting that prenatal hormones may affect later toy preferences.
Aggressive behavior may occur when two (or more) individuals are in conflict, typically over limited resources such as territories, food, and mates. Evidence strongly suggests that androgens mediate aggressive behavior across many species. Seasonal variations in testosterone levels and variations in aggression coincide; aggressive behaviors increase at puberty (when the testes become active and androgens rise); in any given species, males are generally more aggressive than females; and castration (removal of the testes) typically reduces aggression in males, while testosterone replacement therapy restores aggression. Human males are much more physically aggressive than females, and these sex differences appear very early. It is possible that boys are more aggressive than girls because androgens promote aggressive behavior, it could be due to exposure to androgens prenatally, or boys may be encouraged to act in aggressive ways. These three hypotheses are not mutually exclusive, but it is extremely difficult to discriminate among them to account for sex differences in human aggressiveness. In many animal species (mice, rats, hamsters, voles, dogs, and possibly some primates), aggressive behavior is both organized and activated by androgens.
Sexual strategies theory describes the psychology of human mating strategies and the ways in which women and men differ in those strategies. Because differences in parental investment are so large between males and females- men have little necessary investment and consequently don't need to make wise choices, whereas mating decisions for women are much more important (since a minimum of none months carrying the child is required)- women are predicted to be choosier than men in short-term mating. Thus, men will sometimes deceive women for the benefit of short-term sex, and men are more likely than women to lower their mating standards for short-term mating situations. However, when choosing long-term mates, the qualities women and men seek are very similar: mates who are intelligent, kind, understanding, healthy, dependable, honest, loyal, loving, and adaptable. There are nonetheless some universal differences in mate preference- women desire mates who possess resources (and are willing to share them), and men more strongly desire youth and health in women (as both are cues to fertility).
References
Note: These references are specifically those added to the content of this page by Naomi Bahm and do not include citations from the outside sources used. Refer to the text attributions to locate citations for articles from other sources.
Bailey, J.M., Vasey, P.L., Diamond, L.M., Breedlove, S.M., Vilain, E., & Epprecht, M. (2016). Sexual orientation, controversy, and science. Psychological Science in the Public Interest, 17(2), 45-101. doi: 10.1177/1529100616637616
Daly. M., & Wilson, M. (1996). Evolutionary psychology and marital conflict. In Buss, D.M., & Malamuth, N.M. (editors), Sex, Power, Conflict: Evolutionary and Feminist Perspectives. Oxford University Press.
Goldstein, J., Seidman, L.J., Horton, N.J., Makris, N., Kennedy, D.N., Caviness, V.S. Jr., Faraone, S.V., & Tsuang M.T. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex, 11, 490-497.
Jaffe, K.E. (2019). Chapter 6: Primate Behavior and Ecology. In Shook, B., Nelson, K., Aguilera, K.& Braff, L. (editors), Explorations: An Open Invitation to Biological Anthropology. American Anthropological Association, licensed CC BY-NC 4.0 International, ISBN 978-1-931303-63-7
Joel, D., Berman, Z., Tavor, I., Wexler, N., Gaber, O., Stein, Y., Shefi, N., Pool, J., Urchs, S., Margulies, D.S., Liem, F., Hänggi, J., Jäncke, L. & Assaf, Y. (2015). Sex beyond the genitalia: The human brain mosaic. PNAS, 112(50), 15468-15473. https://doi.org/10.1073/pnas.1509654112
Larsen, C.S. (2003). Equality for the sexes in human evolution? Early hominid sexual dimorphism and implications for mating systems and social behavior. Proceedings of the National Academy of Sciences of the United States of America, 100 (16), 9103–9104. https://www.pnas.org/doi/10.1073/pnas.1633678100
LeVay, S. (1991). A difference in hypothalamic structure between heterosexual and homosexual men. Science, 253(5023), 1034-1037. doi: 10.1126/science.1887219
Luders, E., Toga, A.W., & Thompson, P.M. (2014) Why size matters: Differences in brain volume account for apparent sex differences in callosal anatomy. The sexual dimorphism of the corpus callosum. NeuroImage, 84, 820-824. http://dx.doi.org/10.1016/j.neuroimage.2013.09.040
McDowell, M.A., Fryar, C.D., Ogden, C.L.,& Flegal, K.M. (2008). Anthropometric reference data for children and adults: United States, 2003–2006. National Health Statistics Reports, 10 (October 22, 2008), 1-48. https://www.cdc.gov/nchs/data/nhsr/nhsr010.pdf
Swearer Napolitano, S.M. (2008) Relational aggression: Not just a female issue. Journal of School Psychology, 46, 611-616. doi: 10.1016/j.jsp.2008.08.001 [available at https://digitalcommons.unl.edu/edpsychpapers/142/]
van Eijk, L., Zhu, D., Couvy-Duchesne B., Strike, L.T., Lee, A.J., Hansell, N.K., Thompson, P.M., de Zubicaray, G.I., McMahon, K.L., Wright, M.J., & Zietsch, B.P. (2021) Are sex differences in human brain structure associated with sex differences in behavior? Psychological Science, 32(8), 1183-1197. doi: 10.1177/0956797621996664
Additional Resources
Web: Main international scientific organization for the study of evolution and human behavior, HBES
Web: Books and Interviews with David Buss
https://labs.la.utexas.edu/buss/books/
Web: Publications by Buss and colleagues
https://labs.la.utexas.edu/buss/publications/
Journal article: Founders of Evolutionary Psychology
Attributions
- Figures:
- Rooster and hen by John Cudworth, licensed CC BY-NC 2.0, found in NOBA Hormones and Behavior by Randy Nelsen
- Left photo: White-handed Gibbons by Cliff from Arlington, Virginia, USA, CC BY 2.0 via Wikimedia Commons; Middle image: Humans extracted from the Pioneer plaque by NASA; vectors by Mysid, Public domain, via Wikimedia Commons; Right photo: Mountain gorillas by Joachim Huber, CC BY-SA 2.0, via Wikimedia Commons
- SDN-POA, no attribution or license information, found in NOBA Hormones and Behavior by Randy Nelsen
- Graph of sex differences overlap, no attribution or license information, found in NOBA Hormones and Behavior by Randy Nelsen
- Left: Brother and sister, by Amanda Westmont, https://goo.gl/ntS5qx, licensed CC BY-NC-SA 2.0, found in NOBA Social and Personality Development in Childhood by Ross Thompson; Right: Seated girl, licensed CC0 Public Domain, found in NOBA Hormones and Behavior by Randy Nelsen
- Aggressive expression by Riccardo Cuppini, CC BY-NC-ND 2.0, found in NOBA Hormones and Behavior by Randy Nelsen
- Pregnant woman, licensed CC0 Public Domain, found in NOBA Evolutionary Theories in Psychology by David Buss
- Text adapted from:
- Hormones & Behavior by Randy J. Nelson, licensed CC BY-NC-SA 4.0 via Noba Project.
- Evolutionary Theories in Psychology by David M. Buss, licensed CC BY-NC-SA 4.0 via Noba Project.
- Changes: Text (and images) from above two sources pieced together with some modifications, transitions and additional content (particularly in the Preliminary Note, Introduction to Sex Differences, Sex Differences in the Brain, and Sex Differences in Sensory Systems and Cognition sections) added by Naomi I. Gribneau Bahm, PhD., Psychology Professor at Cosumnes River College, Sacramento, CA.


