14.5: Brain Mechanisms and Intelligence
- Page ID
- 113225
This page is a draft and under active development. Please forward any questions, comments, and/or feedback to the ASCCC OERI (oeri@asccc.org).
- Describe the brain structures included in the P-FIT model of the biology of intelligence.
- Describe the Default-Mode Network (DMN) and its relationship to the biology of intelligence.
- Discuss the type of attention that is probably involved when someone is taking an I.Q. test and how this is related to interpretation of brain imaging studies of the brain networks involved in human intelligence.
Overview
According to Hearne, et al. (2016, p.1), "Human intelligence can be broadly defined as the capacity to understand complex ideas, adapt effectively to the environment and engage in complex reasoning1. Measures of intelligence can be related to performance on virtually any cognitive task, from sensory discrimination2 to challenging cognitive tasks such as the identification of patterns in the Raven’s Progressive Matrices test3. Importantly, scores on intelligence tests can accurately predict various life outcomes, including academic success4, job performance5 and adult morbidity and mortality6." In spite of the adaptive importance of intelligence in human life, the brain mechanisms involved in intelligence are not well understood. However, research makes it increasingly clear that complex functions such as cognition and intelligence involve many brain areas acting together in interconnected neural networks comprised of multiple brain areas. Furthermore, interactions between different networks of brain areas are also important if we are to understand how the brain creates cognition and intelligence. Functional magnetic resonance imaging (fMRI) has been used to investigate the relationship between individual differences in intelligence and brain activity during cognitive activities such as working memory and reasoning. As discussed in prior modules of this chapter, brain imaging studies implicate neural interactions in a fronto-parietal network underlying many of the functions associated with human intelligence. These observations have resulted in the influential Parieto-Frontal Integration Theory of intelligence (P-FIT) which identifies a number of interacting brain structures associated with individual differences in human intelligence including frontal and parietal lobes and other structures. Additional research (Hearne, et al., 2016) shows that individual differences in intelligence between people are also associated with the degree of neural interaction between the fronto-parietal network and the default-mode network. Greater connectivity between these two neural networks when the brain is at rest (i.e. in the absence of any specific cognitive task) is correlated with higher intelligence scores in individuals compared to those with lesser connectivity between the fronto-parietal and default mode networks. Interactions with a dorsal attention network (DAN) must also be considered for a more complete understanding of the complex network interactions involved in cognition and intelligence.
Brain Mechanisms and Intelligence
Because of the complexity and number of interacting factors in human intelligence, understanding the brain mechanisms in human intelligence is a monumental task for researchers. The figure below showing Carroll's influential model of human intelligence is a reminder of the large number of interacting abilities involved. The various factors and abilities shown are derived mathematically using factor analysis based on analysis of patterns of correlations in the performances of large numbers of people on measures of intelligence such as standard I.Q. tests (e.g. Wechsler Adult Intelligence Scale, WAIS).
Figure \(\PageIndex{1}\): At the top of Carroll's three-stratum model of human intelligence is the g-factor, general intelligence. Notice the large number of Stratum I abilities which comprise the factors in g and the more specific g-factors in Stratum II. Abbreviations: fluid intelligence (Gf), crystallized intelligence (Gc), general memory and learning (Gy), broad visual perception (Gv), broad auditory perception (Gu), broad retrieval ability (Gr), broad cognitive speediness (Gs), and processing speed (Gt). (Image and abbreviations from Wikimedia Common; File:Carroll three stratum.svg; https://commons.wikimedia.org/wiki/F...ee_stratum.svg; by Victor Chmara; made available under the Creative Commons CC0 1.0 Universal Public Domain Dedication).
In spite of this complexity, neuroscientists using brain imaging methods have identified brain regions associated with differences in measured I.Q. or other measures of general intelligence. As mentioned earlier in this chapter, Jung and Haier (2007) reviewed brain imaging studies and identified a set of brain areas correlated with individual differences in intelligence and reasoning among people. They called this set of interconnected brain regions the fronto-parietal network and dubbed their model the Parietal-Frontal Integration Theory (P-FIT). "The P-FIT model includes, by Brodmann areas (BAs): the dorsolateral prefrontal cortex (BAs 6, 9, 10, 45, 46, 47), the inferior (BAs 39, 40) and superior (BA 7) parietal lobule, the anterior cingulate (BA 32), and regions within the temporal (BAs 21, 37) and occipital (BAs 18, 19) lobes. White matter regions (i.e., arcuate fasciculus) are also implicated" (Jung and Haier, 2007, p. 135).
The arcuate fasciculus interconnects regions of temporal and parietal cortex with frontal cortex (see Figure 14.8.2). This bundle of axons connects Wernicke's and Broca's areas, in temporal and frontal lobes, respectively, which are involved in language comprehension and language production (see modules on language later in this chapter). Surprisingly, this does not mean that language ability is necessary for cognition and intelligence.
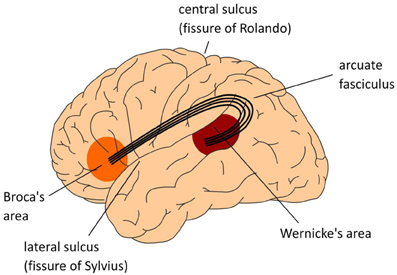
Figure \(\PageIndex{2}\): (Left): The Arcuate Fasciculus. Lateral view of left hemisphere. (Center): The center figure also displays a lateral view of the left hemisphere. The numbers indicate Brodmann areas (BA). These are areas with differences in the cytoarchitectonics (i.e., composition of cell types). The memory areas are in the temporal cortex (in yellow) including the angular gyrus in parietal cortex. Broca's area (Brodmann areas 44 and 45) and adjacent cortex (Brodmann areas 47 and 6) in the frontal lobe involved in language. Control operations recruit another part of the frontal lobe (in pink), and the Anterior Cingulate Cortex (ACC; not shown in the center figure), as well as areas involved in attention. (Right): Medial view of cerebral cortex showing major gyri. (Images from Wikimedia Commons; Left image: File:The classical Wernicke-Lichtheim-Geschwind model of the neurobiology of language fpsyg-04-00416-g001.jpg; https://commons.wikimedia.org/wiki/F...00416-g001.jpg; by Peter Hagoort; licensed under the Creative Commons Attribution 3.0 Unported license. Center image and caption: File:The MUC (Memory, Unification, Control) model of language fpsyg-04-00416-g002.jpg; https://commons.wikimedia.org/wiki/F...00416-g002.jpg; by Peter Hagoort; Hagoort P (2013) MUC (Memory, Unification, Control) and beyond. Front. Psychol. 4:416. doi: 10.3389/fpsyg.2013.00416 http://journal.frontiersin.org/article/10.3389/fpsyg.2013.00416/full); licensed under the Creative Commons Attribution 3.0 Unported license. Right image: File:Medial surface of cerebral cortex - gyri.png; https://commons.wikimedia.org/wiki/F...tex_-_gyri.png; by Patric Hagmann et.al., Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, et al. (2008) Mapping the Structural Core of Human Cerebral Cortex. PLoS Biol 6(7): e159. doi:10.1371/journal.pbio.0060159[1]; licensed under the Creative Commons Attribution 2.5 Generic license).
For example, "patients with even severe damage to the language network can retain high intelligence and the ability to perform challenging cognitive tasks, like arithmetic (Varley et al., 2005) and causal reasoning (e.g., Varley and Siegal, 2000; see Fedorenko and Varley, 2016, for a review)" (Assem, et al., 2020, p. 139). This finding strongly suggests that cognitive tasks like arithmetic and causal reasoning do not depend upon language or language networks. This finding is consistent with the theory that causal reasoning is a component of general intelligence evolved by "genetic internalization" of causality, similarity, and predictive relations into functional organization of the brain, and that general intelligence is found in many non-human animal species even though they lack language (Koenigshofer, 2017).
Using fMRI, Duncan (2010, 2013) has identified a frontal and parietal pattern of neural activity associated with diverse cognitive tasks and general fluid intelligence. Duncan calls this pattern "multi-demand" (MD) because this activation pattern is a "salient part of the brain’s response to many different kinds of cognitive challenge. . . . [T]his multiple-demand (MD) pattern extends over a specific set of regions in prefrontal and parietal cortex, in particular: cortex in and around the posterior part of the inferior frontal sulcus (IFS), [cortex' in the anterior insula and the adjacent frontal operculum (AI/FO), in the pre-supplementary motor area and adjacent dorsal anterior cingulate (preSMA/ACC), and [cortex] in and around the intraparietal sulcus (IPS). A smaller region of accompanying activity is sometimes seen in rostrolateral prefrontal cortex (RPFC)" (Duncan, 2010, p. 172). A similar pattern of activation is seen in tasks associated with general fluid intelligence. Furthermore, "recent lesion data suggest that deficits in fluid intelligence are specifically associated with damage to the MD network (Duncan, 2010, p. 172).
Using fMRI, Gray, et al. (2003) found that individual differences in general fluid intelligence (gF) were associated with differences in activation of attentional mechanisms in the lateral prefrontal and parietal cortex during a working memory task. These results are consistent with the P-FIT model of intelligence and suggest that at least some of the differences in measured intelligence among people may be due to differences in brain networks that mediate attention.
According to Assem, et al. (2020, p. 131), "A distributed frontoparietal Multiple Demand (MD) network [Duncan, 2010, 2013] has long been implicated in intelligent behavior, and its damage has been associated with lower intelligence and difficulties in problem solving. . . [It] has been linked to our ability to engage in goal-directed behaviors, solve novel problems, and acquire new skills. . . . Damage to this network as a result of stroke, degeneration or head injury leads to poorer executive abilities (attention, working memory, and inhibitory control) and lower fluid intelligence (Glascher et al., 2010; Roca et al., 2010; Woolgar et al., 2010) . . . and aberrant functioning of this network has been reported in a variety of neurological and psychiatric disorders."
Emphasizing interactions between various brain networks in human intelligence, Hearne, et al. (2016, p. 7) state: "How the brain self-reorganizes to achieve optimal configurations of functional networks across individuals with varying levels of intelligence is an open question. Recent neuroimaging work has suggested that transient cooperation between different neural systems, including fronto-parietal, cingulo-opercular and default-mode networks, is integral to complex cognitive tasks such as reasoning42,43, memory recollection44 and working memory performance39,45. Future studies should test the notion that individual differences in intelligence rely on dynamic, context-specific, reconfigurations of local activity and connectivity within a diffuse system comprising fronto-parietal, cingulo-opercular and default-mode regions46."
Hearne, et al. (2016, p. 1) "revealed a novel contribution of across-network interactions between default-mode and fronto-parietal networks to individual differences in intelligence at rest (i.e. in the absence of any specific cognitive task). Specifically, [they] found that greater connectivity in the resting state was associated with higher intelligence scores." The default-mode network includes the medial prefrontal cortex, posterior cingulate cortex, and the inferior parietal lobule. Other structures sometimes considered part of this network are the middle temporal lobe and the precuneus.
What is the significance of connectivity between networks at rest, when no specific cognitive task is being performed? The answer to this question reflects another method used by neuroscientists to understand the functional and neuroanatomical relationships between networks of interconnected brain areas. Dixon et al. (2017) describe the method this way: "Resting state functional connectivity has emerged as a powerful, non-invasive tool for delineating the functional network architecture of the human brain." In other words, connectivity between brain networks at rest helps neuroscientists determine how various brain networks are "wired" together and how they functionally interact with one another to produce cognition and intelligence.
In this light, remember that Hearne, et al. (2016, p.1) found that "greater connectivity in the resting state [between default-mode and fronto-parietal networks] was associated with higher intelligence scores." To help us understand why this might be so, we have to know something about the suspected role that the default-mode network plays in cognition. Dixon, et al. (2017, p.632) explain it this way: "The default network (DN) is involved in a variety of internally-directed processes, including self-reflection, autobiographical memory, future event simulation, conceptual processing, and spontaneous cognition . . . and exhibits decreased activation during many cognitive tasks that demand external perceptual attention." The finding of decreased activation during cognitive tasks requiring attention to input from the external environment suggested to some researchers that there might be two competing mutually inhibiting networks, one for internal reflection, and the other, a "task positive" or "task-related" network for attention to the external world. However, later research found "co-activation and positive functional connectivity between the DN and the frontoparietal control network (FPCN)―a component of the “task positive” network―during some task conditions, including mind wandering, spontaneous thought, autobiographical future planning, creativity, memory recall, working memory guided by information unrelated to current perceptual input, social working memory, and semantic decision making. . . . On the other hand, studies have generally found anticorrelation [increase in activity in one network is associated with a decrease in the other] between the DN and other components of the “task positive” network, particularly the dorsal attention network (DAN)" (Dixon, et al., 2017, p. 633).
The “dorsal attention network” (DAN) or “frontoparietal network” directs visual attention and short-term memory processes . . . Moreover, this network is distinct from a cingulo-opercular cognitive control network (CCN). Yet, no consensus has been reached regarding the precise components of the DAN. On the basis of task-based and resting-state fMRI studies, the DAN in humans is typically defined to include all or some of the following four regions: (1) intraparietal sulcus (IPS)/ superior parietal lobule; (2) superior pre-central sulcus (sPCS) containing the homolog of primate frontal eye fields; (3) inferior pre-central sulcus (iPCS), alternately known as inferior frontal junction; and (4) the motion-sensitive area MT complex (MT) . . . [Moreover,] subcortical structures, such as superior colliculus and pulvinar, are often implicated in attentional functions" and parts of the cerebellum are also involved in the DAN" (Brissenden, et al., 2016, p. 6083-4).
Figure \(\PageIndex{3}\): (Left): The Default Mode Network, midsagittal and horizontal cross-section views. (Right): Default Mode Network contrasted with Task-Related Network. On a green background, the default mode network is highlighted in warm colors (red and yellow) and the task-related network is highlighted in cold colors (blue and light blue). Top, medial views. Bottom, lateral views. (Images from Wikimedia Commons; Left: File:Default mode network-WRNMMC.jpg; https://commons.wikimedia.org/wiki/F...ork-WRNMMC.jpg; by John Graner, Neuroimaging Department, National Intrepid Center of Excellence, Walter Reed National Military Medical Center; in the public domain in the United States because it is a work prepared by an officer or employee of the United States Government as part of that person’s official duties. Right: File:Default mode and task-related maps for healthy subjects.jpg; https://commons.wikimedia.org/wiki/F...y_subjects.jpg; by Shim G, Oh JS, Jung WH, et al., Shim G, Oh JS, Jung WH, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behavioral and Brain Functions : BBF. 2010;6:58. doi:10.1186/1744-9081-6-58. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2959003/; Shim G, Oh JS, Jung WH, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behavioral and Brain Functions : BBF. 2010;6:58. doi:10.1186/1744-9081-6-58. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2959003/).
"[A]ttention is a selection mechanism that serves to choose a particular source of stimulation, internal train of thoughts, or a specific course of action for priority processing . . . However, in certain situations attention is necessary to supervise goal-directed action . . . [and] is necessary for detecting errors, and controlling behavior in dangerous and novel or unpracticed conditions. Thus, attention mechanisms are also central to the generation of voluntary behavior, which often involves inhibition of automatic responses. . . . [Furthermore, attention can be] mostly driven by external stimulation or . . . [by] endogenous processes such as voluntary intentions or expectations. . . . [or] attention can also be directed to an object because of its relevance to our current goals" (Rueda, et al., 2015, p.183). Additionally, sustained attention on a task (such as taking an I.Q. test) must be maintained during distraction. Sustained, focused attention is clearly involved in a person's performance on many measures of intelligence. Rueda, et al. (2015) identify three types of attention networks: 1) alerting, such as attention to a sudden loud noise (mediated by norepinephrine); 2) orienting, shifts of attention from one location to another, involving parietal and frontal cortices and which uses acetylcholine; and 3) executive attention, attention required to deal with distraction and incongruous (conflicting) information especially if incompatible with the task, goal, or problem at hand; executive attention involves anterior cingulate and prefrontal cortices, and is modulated by levels of brain dopamine and serotonin.
The close association of attention with ability to concentrate during working memory and problem solving raises the possibility of a confound between attention and intelligence in some of the brain imaging studies supporting the P-FIT and similar models of the biology of intelligence discussed above. In other words, the networks identified with intelligence may turn out to be more accurately defined as attention networks. If so, some of the brain imaging research in intelligence may be accessing attentional networks, rather than intelligence networks. In this case, variations in I.Q. associated with the functioning of these networks may primarily reflect differences among people in attentional mechanisms which then affect performance on I.Q. tests. Attention and working memory, though certainly essential to problem solving, reasoning, and intelligent action, do not capture the reasoning processes themselves. When interpreting research in this area, it is important to be aware of the specific features of the cognitive tasks under study. For example, does any particular imaging study use control procedures that can separate effects of attention and working memory from the reasoning processes of intelligence themselves? It may turn out that this separation is so difficult to accomplish that, in actual practice, the overlap between attention networks and intelligence networks may be hard to tease out. However, attentional networks have been identified such as the dorsal attention network (DAN) and a cingulo-opercular cognitive control network (CCN) (and others mentioned above), and the extent to which these do not overlap the networks associated with general fluid intelligence, researchers may be closer to identifying networks involved in reasoning and intelligence as distinct from the mechanisms of attention.
In the meantime, we do know that variations among people in I.Q. and other measures of intelligence are correlated with differences in their brain activity in the fronto-parietal network (as proposed by the P-FIT model and Duncan's MD network), and with the degrees of functional connectivity at rest between this network and the default-mode network. However, additional research is needed to clarify the underlying reasons for these correlations between measures of intelligence and levels of activity in these brain networks and their connectivity.
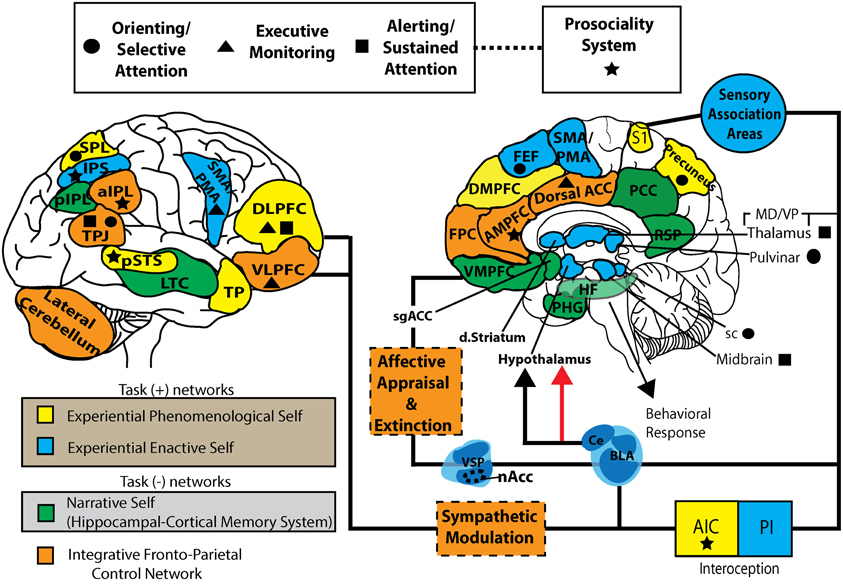
Figure \(\PageIndex{4}\): One model of the complex interactions between various brain areas involved in cognitive control of behavior generating various neural models of the intelligent self in interaction with the internal (mental) and external (sensory) worlds (the foregoing caption by the author of this module, Kenneth A. Koenigshofer, Ph.D.). (The following caption is from the authors of this image). This working model represents a parcellation of task positive (self-specifying: EPS and EES), task negative (NS), and integrative fronto-parietal control networks. EPS, experiential phenomenological self; EES, experiential enactive self; NS, narrative self; FPCN, fronto-parietal control network; FEF, frontal eye fields; DMPFC, dorsal-medial prefrontal cortex; AMPFC, anterior medial prefrontal cortex; VMPFC, ventromedial prefrontal cortex; PHG, parahippocampal gyrus; HF, hippocampal formation; RSP, retrosplenial cortex; PCC, posterior cingulate cortex; Dorsal ACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; TP, temporal pole, LTC, lateral temporal cortex; TPJ, temporoparietal junction; sPL, superior parietal lobe; pIPL, posterior inferior parietal lobe; aIPL, anterior inferior parietal lobe; nAcc, nucleus accumbens; VSP, ventrostriatal pallidum; dstriatum, dorsal striatum; S1, primary somatosensory cortex; AIC, anterior insular cortex; PIC, posterior insular cortex; sgACC, subgenual anterior cingulate cortex; VMpo, ventromedial posterior nucleus; sc, superior colliculus; BLA, basolateral amygdala; CE, central nucleus. (Image from Wikimedia Commons; File:S-ART Mindfulness and brain1.jpg; https://commons.wikimedia.org/wiki/F...and_brain1.jpg; by Vago DR and Silbersweig DA; licensed under the Creative Commons Attribution 3.0 Unported license).
Cellular Basis of Differences in Human Intelligence
Most of the research on the neurological basis of intelligence in humans has focused either on 1) gene loci associated with individual differences in intelligence or 2) is based on using whole brain imaging to identify brain regions correlated with individual differences in intelligence. Some studies show common genes for differences in brain volume and differences in intelligence, while others suggest that genes that facilitate growth of neurons are associated with higher IQ (see section below on genes and intelligence). Functional and structural MRI studies highlight correlations between specific areas of cortex, including frontal and temporal lobes, and measures of g, general intelligence (see discussion of g in prior modules in this chapter). Higher order association cortex in frontal and temporal lobes contains large numbers of pyramidal neurons with large and complex dendritic branching that might account for individual differences in cortical thickness, synaptic integration, and perhaps IQ (Goriounova, et al., 2018). These neurons and their connections are "the principal building blocks for coding, processing, and information storage in the brain and give rise to cognition (Salinas & Sejnowski, 2001). Given their vast number in the human neocortex, even the slightest change in efficiency of information transfer by neurons may translate into large differences in mental ability" (Goriounova, et al., 2018, p. e41715).
As shown in Figure 14.5.5 (below), Goriounova, et al. (2018) found that IQ scores are positively correlated with cortical thickness of the temporal lobe, the length of pyramidal neuron dendrites, and complexity of dendritic branching. "Thus, larger and more complex pyramidal neurons in temporal association area may partly contribute to thicker cortex and link to higher intelligence" (p. e41721).
These same researchers also found evidence that larger temporal lobe pyramidal neurons generated action potentials faster than smaller ones and that faster action potential (AP) onset generates better temporal tracking of high frequency inputs leading to more efficient information coding and transfer in larger temporal lobe pyramidal neurons. They report that "human cortical pyramidal neurons from individuals with higher IQ scores generate faster APs" and therefore "neurons from individuals with higher IQ scores are better equipped to process synaptic signals at high rates and at faster time scales, which is necessary to encode large amounts of information accurately and efficiently" (Goriounova, et al., 2018, p. e41724). These researchers state that their results help explain why faster reaction times in even simple tasks (as a measure of mental processing speed) are consistently associated with higher general intelligence measured by IQ scores on intelligence tests and other measures of Spearman's g (see discussion of g, general intelligence factor, in prior modules of this chapter).
They also note that "Pyramidal cells are integrators and accumulators of synaptic information. Larger dendrites can physically contain more synaptic contacts and integrate more information. Indeed, human pyramidal neuron dendrites receive twice as many synapses as in rodents (DeFelipe et al., 2002) and corticocortical whole-brain connectivity positively correlates with the size of dendrites in these cells" (Goriounova, et al., 2018, p. e41726). Goriounova et al. (2018) claim that larger and more complex dendritic arrays in larger pyramidal neurons are accompanied by increases in integration in cortical areas. "Human pyramidal cells in layers 2 and 3 [of the cerebral cortex] have 3-fold larger and more complex dendrites than in macaque or mouse" which "suggest evolutionary pressure on both dendritic structure and AP waveform and emphasize specific adaptations of human pyramidal cells in association areas for cognitive functions" (p. e41727). They reason, therefore, that larger pyramidal neurons, with more extensive dendritic branching and faster onset of action potentials, are necessary for higher order cortical processing associated with human cognition. Based on this conclusion, they suggest that differences among individuals in "neuronal complexity" might associate with differences among people in mental ability (Figure 14.8.5).
Figure \(\PageIndex{5}\): A cellular basis of human intelligence. Higher IQ scores associate with larger dendrites, faster action potentials during neuronal activity and more efficient information tracking in pyramidal neurons of temporal cortex. The figure is based on the results from Goriounova et al.(2018). (Image from Wikimedia Commons; File:Cellular basis of IQ.png; https://commons.wikimedia.org/wiki/F...asis_of_IQ.png; by Natalia A. Goriounova and Huibert D. Mansvelder; licensed under the Creative Commons Attribution 4.0 International license).
Summary of Other Brain Features Correlated with Differences in Human Intelligence
The following table summarizes characteristics of the brain associated with individual differences among humans in general intelligence, as measured by IQ and other psychological tests of intelligence. Based on Goriounova and Mansvelder (2019).
============================================================================================
Table 14.5.1: Neurological features (unshaded rows) associated with intelligence (rows 3 and 4 are continuations of rows 1 and 2).
=============================================================================================
| NEUROLOGICAL TRAIT | overall brain volume | cortical thickness in frontal, parietal and temporal association cortex | rapid increase in cortical thickness in childhood with rapid decrease in cortical thickness by early adolescence as pruning of excess synapses occurs increasing processing efficiency | efficiency of processing in distributed cortical areas (frontal, parietal, temporal) as measured by lower neural activity and lower brain metabolic energy required for mental task performance by high I.Q. individuals |
| CORRELATION OF NEUROLOGICAL TRAIT WITH INTELLIGENCE AS MEASURED BY PSYCHOLOGICAL TESTS (r =, when stated by Goriounova & Mansvelder) | positive, r= between .24 to .33 (correlated with overall brain volume) |
positive correlations with IQ (top of this column above, i.e. cortical thickness) |
both changes (rapid increase followed by rapid decrease) in cortical thickness positively correlated with high I.Q. | positive correlations with general "fluid" intelligence |
| NEUROLOGICAL TRAIT (continued) | cortical structure and cortical thickness in lateral areas of temporal lobes and in temporal pole | prefrontal cortex: structure, function and connectivity | functional integrity of white matter (pathways composed of myelinated axons interconnecting brain regions), especially between right frontal and temporal cortex; mental retardation associated with severe damage to white matter | left hemisphere gray matter volume and white matter (myelinated axons) connectivity between left posterior orbital frontal cortex (OFC) and rostral anterior cingulate cortex (rACC) |
| CORRELATION OF NEUROLOGICAL TRAIT WITH INTELLIGENCE AS MEASURED BY PSYCHOLOGICAL TESTS (continued) | positive correlation with general "crystallized" intelligence dependent on verbal abilities, semantic working memory and acquired knowledge (correlated with structure and cortical thickness of temporal lobes and temporal pole) | positive correlation especially with reasoning ability (i.e. correlated with prefrontal structure, function, and connectivity) | positive correlation with intelligence (i.e. correlated with white matter integrity) | positive correlations with general "fluid" intelligence, "g" (see section 18.11) |
============================================================================================
Summary Conclusions from Table 14.5.1 (above): "Conclusions on Gross Brain Distribution of Intelligence: intelligence is supported by a distributed network of brain regions in many, if not all, higher-order association cortices, also known as parietal-frontal network (Jung and Haier, 2007). This network includes a large number of regions—the dorsolateral prefrontal cortex, the parietal lobe, and the anterior cingulate, multiple regions within the temporal and occipital lobes and, finally, major white matter tracts. Some limited division of function can be observed, implicating frontal and parietal areas in fluid intelligence, temporal lobes in crystallized intelligence and white matter integrity in processing speed. Although brain imaging studies have identified anatomical and functional correlates of human intelligence, the actual correlation coefficients have consistently been modest, around 0.15–0.35" (Goriounova and Mansvelder, 2019). Note that the amount of variation (variance) in a variable accounted for by its correlation with another variable is equal to the correlation coefficient squared. For example, in this case, a correlation of 0.35 means that only .35 x .35 = .1225 (12.25%) of the variation in intelligence among individuals studied is attributed to its correlation with anatomical and functional features of the brains studied in brain imaging studies, according to Goriounova and Mansvelder (2019). Thus, in the best case, they find that about 88% of the variance in intelligence among individuals studied is accounted for by observed differences among individuals in anatomical and functional features of the brains of those studied using brain imaging. Perhaps the brain imaging is detecting brain regions involved in only some components of intelligence (perhaps attention?) while missing others such as processing of similarity or causal relations, for example.
Genes and Intelligence
Attempts to find single genes that determined intelligence completely failed. The conclusion was that intelligence must be a highly polygenic trait. However, most of the genes that affect intelligence are in non-coding regions of DNA that do not code for protein structure but are instead regulatory genes (which turn on and off other genes) that are involved in generation of cortical neurons during brain development. Different genes correlated with intelligence primarily affect prenatal brain developmental processes including "the proliferation of neural progenitor cells and their specialization, the migration of new neurons to the different layers of the cortex, the projection of axons from neurons to their signaling target and dendritic sprouting" (Goriounova and Mansvelder, 2019).
Genes also affect cell-cell interactions. "Many of the identified genes that play a role in neurodevelopment might contribute to synaptic function and plasticity. Brain function relies on highly dynamic, activity-dependent processes that switch on and off genes. These can lead to profound structural and functional changes and involve formation of new and elimination of unused synapses, changes in cytoskeleton, receptor mobility and energy metabolism. Cognitive ability may depend on how efficient neurons can regulate these processes . . . some candidate genes . . . are specifically involved in axon guidance during neuronal development" (Goriounova and Mansvelder, 2019). This may suggest that efficiency in connections between different circuits and brain areas may be a significant factor in intelligence. Axons that don't go to the right places during brain development would lead to processing inefficiencies.
Other genes that may be involved in differences among individuals in intelligence affect signaling pathways involved in neuron proliferation and migration (during prenatal brain development; see section in Chapter 4 on development of the nervous system) and synaptic communication throughout development.
Other genes that may be involved in differences in intelligence among people are genes that organize pre-synaptic neuron activities especially synaptic vesicles and their release of transmitter.
Others regulate cAMP and CREB involved in gene transcription that for neurons plays a role in synaptic plasticity, learning and memory.
Still others play a role in voltage-gated calcium channel function. Recall that calcium channels open when an action potential reaches an axon ending stimulating movement and binding of synaptic vesicles to the pre-synaptic axonal membrane followed by transmitter release.
Interestingly, most of the energy consumed by the brain (20% of the body's total) is for generation of post-synaptic potentials (PSPs). "Notably, the emergence of higher cognitive functions in humans during evolution is also associated with the increased expression of energy metabolism genes" and "cognitive ability associates with genetic variation in several genes that code for regulators of mitochondrial function" essential to energy metabolism.
"In addition, genes involved in lipid metabolism (BTN2A1 and BTN1A1) and glucose and amino acid metabolism (GPT) . . . [as well as] "microtubule-associated proteins [which] . . . affect recycling of synaptic receptors and neurotransmitter release . . . linked to intelligence by several studies . . . are among the candidate genes of intelligence" (Goriounova and Mansvelder, 2019). The genes affecting microtubule-associated proteins are known to be altered in Alzheimer's, Parkinson's, and Huntington's Disease.
The highest expression of the genes associated with intelligence occur "within pyramidal neurons in hippocampal area CA1 and cortical somatosensory regions . . . [and significant expression] in medium spiny neurons. . . . Pyramidal neurons are the most abundant neuronal types in neocortex and hippocampus, structures associated with higher executive functions, decision-making, problem-solving and memory. Striatal medium spiny neurons constitute 95% of all neuronal types within the striatum, a structure responsible for motivation, reward, habit learning and behavioral output" (Goriounova and Mansvelder, 2019).
A Central Role for Pyramidal Neurons and Implications of Cross-Species Comparisons
According to Goriounova and Mansvelder (2019), "Genetic studies indicate that expression of genes associated with intelligence accumulates in cortical pyramidal neurons (Savage et al., 2018; Coleman et al., 2019). Comparisons of key cellular properties of pyramidal neurons across species may offer insights into functional significance of such differences for human cognition . . . compared to rodents and macaques, human layer 2/3 pyramidal cells have threefold larger and more complex dendrites (Mohan et al., 2015). Moreover, these large dendrites also receive two times more synapses than rodent pyramidal neurons (DeFelipe et al., 2002). Apart from structural differences, human pyramidal neurons display a number of unique functional properties. human excitatory synapses recover 3–4 times faster from depression than synapses in rodent cortex, have more speedy action potentials and transfer information at up to nine times higher rate than mouse synapse (Testa-Silva et al., 2014)." In humans, larger pyramidal neurons, with longer dendrites, more complex dendritic branching, more synaptic connections, and faster onset of action potentials, combine to make possible the processing of greater amounts of information, with greater efficiency and integration among brain areas, than in other animals, and these large neurons may play a central role in the vast differences between human and non-human animal intelligence (see Figure 14.8.5).
Brain Correlates of Creativity
Jung and Haier (2013) report a number of brain correlates of intelligence and creativity. However, they argue against the idea of one brain area for one cognitive function. Instead, as discussed in the prior module, they argue that brain networks involving multiple brain areas are involved in cognition, especially in complex psychological processes such as intelligence and creativity. Nevertheless, they recognize that brain injury and lesion studies reveal brain structures that are necessary, though not sufficient, for certain psychological functions. They give three examples: 1) Phineas Gage, who survived an iron rod passing through his frontal lobe resulting in personality and emotional changes as well as impaired judgement and loss of many social inhibitions; 2) "Tan," whose brain damage led to identification of Broca's area for language expression; and 3) H.M., whose bilateral surgical removal of temporal lobe structures including hippocampus revealed the role of hippocampus and related structures in formation of new long-term explicit memories and their retrieval.
Within this context, Jung and Haier (2013) note some interesting observations from post-mortem examination of the brain of the famous theoretical physicist, Albert Einstein (whose work led to the equation, E=mc2), and what it might suggest about brain mechanisms in creativity. Einstein's brain was unremarkable in many ways. Its size and weight were within the normal range for a man of his age, and frontal and temporal lobe morphology and corpus callosum area were no different from control brains. However, there was one pronounced difference. According to Jung and Haier, Einstein's brain was missing the parietal operculum, the typical location of the secondary somatosensory cortex, resulting in a larger inferior parietal lobule. In Einstein's brain, the inferior parietal lobule was approximately 15% wider than in the brains of normal controls. According to Jung and Haier, this region of brain is associated with "visuospatial cognition, mathematical reasoning, and imagery of movement . . . and its expansion was noted in other cases of prominent physicists and mathematicians." They add that further examination of this area of Einstein's brain revealed that rather than more neurons, this region of his brain had a much larger number of glial cells, which provide nutrition to neurons, perhaps indicating an unusually large amount of activity among neurons in this region of his brain.
Significantly, as described in the prior module, parietal cortex has strong linkages with prefrontal cortex forming a frontoparietal network: the inferior parietal lobule is primarily connected with dorsolateral prefrontal cortex (Bruner, 2010), associated, in part, with abilities for abstract thought, while upper parietal regions, according to Bruner, as discussed in module 14.2, are associated in the literature with functions such as abstract representation, internal mental images, “imagined world[s],. . . and thought experiment” (i.e., imagination). Jung and Haier detail another study of Einstein's right prefrontal association cortex, where researchers found greater packing density of neurons (same number of neurons in a smaller space), which was interpreted as shorter conduction times between cortical neurons in Einstein's brain compared to control brains. Jung and Haier conclude that Einstein's brain differed from controls in the frontoparietal network. These authors have proposed that the frontoparietal network is crucial to human intelligence; furthermore they hypothesized that differences among people in the efficiency of neural communication between the frontal and parietal regions of cortex accounts for differences in intelligence in humans (Jung & Haier, 2007). In part, this idea is based on their finding that high IQ people show less activity in these brain areas during a complex cognitive task, while lower IQ people show more brain activity, suggesting that high IQ is related to efficiency in neural information processing operations. Moreover, higher IQ and ability for abstraction are both inversely correlated with cerebral glucose metabolic rate (Haier et al., 1988, 1992, 2003, 2004), suggesting an efficiency model of individual differences in g in which superior ability for abstraction increases processing efficiency. In their Parietal-Frontal Integration Theory (P-FIT) of the neural basis of intelligence, after sensory processing, information "is then fed forward to the angular, supramarginal, and inferior parietal cortices, wherein structural symbolism and/or abstraction are generated and manipulated. The parietal cortex then interacts with frontal regions that serve to hypothesis test various solutions to a given problem." They add that "the anterior cingulate is involved in response selection as well as inhibition of competing responses. This process is critically dependent on the fidelity of underlying white matter needed to facilitate rapid and error-free transmission of data between frontal and parietal lobes" (Jung & Haier, 2013, p. 239). They also note that research in genetics shows that "intelligence and brain structure (i.e., gray and white matter) share common genes" (p. 240).
Regarding creativity specifically, these authors refer to a theory by Flaherty (2005) which proposes a frontotemporal system driven by dopaminergic limbic activity which provides the drive for creative expression whether art, music, writing, science, etc. and as measured by tests of divergent thinking. Jung and Haier (2013) explain that the temporal lobe normally inhibits the frontal lobe so that lesion or mild dysfunction of the temporal lobe releases activity from the frontal lobe by disinhibition causing increased interactions of frontal lobe with other brain regions, sometimes leading to increased creative outputs from neurological patients with left side damage. They argue that this and other data from "three structural studies point to a decidedly left lateralized, frontosubcortical, and disinhibitory network of brain regions underlying creative cognition and achievement" (p. 244). They add that this model, which still requires much more empirical investigation, "appears to include the frontal and temporal lobes, with cortical “tone” being modulated via interactions between the frontal lobes, basal ganglia and thalamus (part of the dopamine system) through white-matter pathways" (p. 244). Although this model is speculative for such a complex form of cognition as creativity, it can guide continuing research into how humans develop creative intellectual and artistic products.
| Inferior parietal lobule | |
|---|---|
|
Figure \(\PageIndex{6}\): Lateral surface of left cerebral hemisphere. Inferior parietal lobule is shown in orange. (Image from Wikimedia Commons; File:Gray726 inferior parietal lobule.png;https://commons.wikimedia.org/wiki/F...tal_lobule.png; by Gray, vectorized by Mysid, colourd by was_a_bee.; this work is public domain. This applies worldwide). |
|
|
Figure \(\PageIndex{7}\): Superficial anatomy of the inferior parietal lobule. Purple: Supramarginal gyrus. Blue: Angular gyrus. LS: Lateral sulcus (Sylvian fissure). CS: Central sulcus. IPS: Intraparietal sulcus. STS:Superior temporal sulcus. PN: Preoccipital notch. (Image and caption from Wikimedia Commons; File:Superficial anatomy of the inferior parietal lobule (IPL).png); https://commons.wikimedia.org/wiki/F...bule_(IPL).png; by Joshua D. Burks Lillian B. Boettcher Andrew K. Conner Chad A. Glenn Phillip A. Bonney Cordell M. Baker Robert G. Briggs Nathan A. Pittman Daniel L. O'Donoghue Dee H. Wu Michael E. Sughrue; licensed under the Creative Commons Attribution 4.0 International license). |
Intelligence Testing and Conceptions of Human Intelligence
The development of tests to measure intelligence has had a major impact on the development of ideas about the nature and structure of human intelligence, and its biological basis in the brain. Most theories of human intelligence are based on data derived from intelligence tests, data which is analyzed using factor analysis, a mathematical method for analyzing patterns of correlations among different measures of mental abilities. In section 14.2, we have already discussed how this method, invented and used by Spearman (1904), revealed the "g" factor in human intelligence.
To understand current thinking and research about the biological basis of human intelligence, it is essential to gain at least a general familiarity with the major theoretical models of human intelligence psychologists have developed. For this purpose, this chapter has a supplementary section entitled, "Traditional Models of Human Intelligence." It is highly recommended that students read that supplementary section. The theories we examine in that section are based to a large extent on intelligence testing and factor analysis, while others are more intuitive. That section also introduces key historical figures, major theories of intelligence, and common assessment strategies used to measure human intelligence.
In section 14.2, we discussed a number of enduring, across-generation, universal regularities of the environment which have been incorporated by evolution into brain organization and intelligence. As described in that section, these enduring facts about how the world works include innate, genetically internalized information about objects in three-dimensional space, the passage of time, daily cycles of light and dark, causality relations (forming basis for causal logic and inference), similarity relations (leading to category formation and categorical logic and inference), and predictive relations, based on covariation of events, allowing human and animal brains to mentally project the organism into future time. All of these invariant properties of the world must be included in the brain's neural models or cognitive maps of the world if the brain is to effectively guide adaptive behavior.
When we examine the traditional models of intelligence in the above referenced supplementary section, you will recognize that each focuses on only one, or a few of the facets of intelligence discussed in the evolutionary approach taken in section 14.2. In a sense, each theory discussed in that section is akin to the fable of the blind men trying to describe an elephant. Each blind man only knows that part of the elephant which he happens to feel and so each man has a different and incomplete understanding of the whole. Likewise, each theory of intelligence focuses on only part of the complex of processes that we collectively refer to as "intelligence." Nevertheless, each theory makes a contribution, and each, in one or more ways, is related to the evolutionary discussion in section 14.2.
For example, as you will see, emotional intelligence, including Gardner's intra- and inter-personal intelligence, is related to neural representations of the contingencies of the social environment--brain mechanisms for which are the focus of the new field of social cognitive neuroscience. Gardner's multiple intelligences include spatial intelligence related to representation of objects in three-dimensional space, abilities which require portions of parietal cortex and hippocampus. At Level III in Carroll's theory of intelligence is "g," general intelligence, related to representations of causal, similarity, and predictive relations, likely involving the frontoparietal network (Jung & Haier, 2007). Of these theories, the first, Carroll's three-stratum theory of human intelligence is by far the most widely accepted and most productive in terms of explanatory power and empirical evidence. With this background, you will be better prepared to understand the traditional models of intelligence and, perhaps more importantly for this course, you will gain a better understanding of the biological bases of intelligence and thinking, the primary focus of this chapter.
References
Assem, Moataz, Blank, Idan A, Mineroff, Zachary, Ademoğlu, Ahmet and Fedorenko, Evelina. (2020). "Activity in the fronto-parietal multiple-demand network is robustly associated with individual differences in working memory and fluid intelligence." Cortex, 131.
Brissenden, J. A., Levin, E. J., Osher, D. E., Halko, M. A., & Somers, D. C. (2016). Functional evidence for a cerebellar node of the dorsal attention network. Journal of Neuroscience, 36(22), 6083-6096.
Dixon, M. L., Andrews-Hanna, J. R., Spreng, R. N., Irving, Z. C., Mills, C., Girn, M., & Christoff, K. (2017). Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage, 147, 632-649.
Duncan, J. (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in cognitive sciences, 14 (4), 172-179.
Duncan, J. (2013). The Structure of Cognition: Attentional Episodes in Mind and Brain. Neuron 80:35–50. Available at: http://dx.doi.org/10.1016/j.neuron.2013.09.015
Goriounova, N. A., Heyer, D. B., Wilbers, R., Verhoog, M. B., Giugliano, M., Verbist, C., ... & Mansvelder, H. D. (2018). Large and fast human pyramidal neurons associate with intelligence. Elife, 7, e41714.
Goriounova, N. A., & Mansvelder, H. D. (2019). Genes, cells and brain areas of intelligence. Frontiers in human neuroscience, 44.
Gray, J. R., Chabris, C. F., & Braver, T. S. (2003). Neural mechanisms of general fluid intelligence. Nature neuroscience, 6 (3), 316-322.
Haier, R. J., Siegel Jr, B. V., Nuechterlein, K. H., Hazlett, E., Wu, J. C., Paek, J., Browning, H.L. and Buchsbaum, M. S. (1988). Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence, 12 (2),199-217.
Haier, R. J., Siegel, B., Tang, C., Abel, L., and Buchsbaum, M. S. (1992). Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence 16 (3), 415.
Haier, R. J., White, N. S., and Alkire, M. T. (2003). Individual differences in general intelligence correlate with brain function during nonreasoning tasks. Intelligence, 31(5), 429-441.
Haier R.J, Jung R., Yeo R., Head K., Alkire M.T. (2004). Structural brain variation and general intelligence. NeuroImage, 23(1), 425-433.
Hearne, L. J., Mattingley, J. B., & Cocchi, L. (2016). Functional brain networks related to individual differences in human intelligence at rest. Scientific reports, 6 (1), 1-8.
Jung, R. E., & Haier, R. J. (2007). The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and brain sciences, 30 (2), 135-154.
Jung, R. E., & Haier, R. J. (2013). Creativity and Intelligence: Brain Networks That Link and Differentiate the Expression of Genius
Koenigshofer, K. A. (2017). General Intelligence: Adaptation to Evolutionarily Familiar Abstract Relational Invariants, Not to Environmental or Evolutionary Novelty. The Journal of Mind and Behavior, 38 (2), 119-153.
Rueda, M. R., Pozuelos, J. P., & Cómbita, L. M. (2015). Cognitive neuroscience of attention from brain mechanisms to individual differences in efficiency. AIMS Neuroscience, 2 (4), 183-202.
Salinas E. & Sejnowski TJ. (2001). Correlated neuronal activity and the flow of neural information. Nature Reviews Neuroscience 2:539–550. DOI: https://doi.org/10.1038/35086012, PMID: 11483997
Spearman, C. (1904). "General Intelligence," Objectively Determined and Measured. The American Journal of Psychology, 15(2), 201-292.
Attributions
"Brain Mechanisms and Intelligence," written by Kenneth A. Koenigshofer, Ph.D., is licensed under CC BY 4.0.



.png?revision=1&size=bestfit&width=465&height=400)