16.1: The Neurological Bases of Emotions
- Page ID
- 130126
This page is a draft and under active development. Please forward any questions, comments, and/or feedback to the ASCCC OERI (oeri@asccc.org).
- Identify what general and specific brain parts are involved in emotions.
- Analyze the anatomical and chemical bases of basic emotions.
- Analyze the biopsychological methods of studying emotions.
- Evaluate the functions of emotions.
Overview
There is a strong connection between brain/body and emotions/affective states. Human and animal research findings have shown brain networks and associated neurotransmitters involved in basic emotion/affective systems.
Affective Neuroscience: What is it?
Affective neuroscience examines how the brain creates emotional responses. Emotions are psychological phenomena that involve changes to the body (e.g., facial expression), changes in autonomic nervous system activity, feeling states (subjective responses), and urges to act in specific ways (motivations; Izard, 2010). Affective neuroscience aims to understand how matter (brain structures and chemicals) creates one of the most fascinating aspects of the mind, the emotions. Affective neuroscience uses unbiased, observable measures that provide credible evidence to other sciences and laypersons on the importance of emotions. It also leads to biologically based treatments for affective disorders (e.g., depression).
The human brain and its responses, including emotions, are complex and flexible. In comparison, nonhuman animals possess simpler nervous systems and more basic emotional responses. Invasive neuroscience techniques, such as electrode implantation, lesioning, and hormone administration, can be more easily used in animals than in humans. Human neuroscience must rely primarily on noninvasive techniques such as electroencephalography (EEG) and functional magnetic resonance imaging (fMRI), and on studies of individuals with brain lesions caused by accident or disease. Thus, animal research provides useful models for understanding affective processes in humans. Affective circuits found in other species, particularly social mammals such as rats, dogs, and monkeys, function similarly to human affective networks, although nonhuman animals’ brains are more basic.
In humans, emotions and their associated neural systems have additional layers of complexity and flexibility. Compared to animals, humans experience a vast variety of nuanced and sometimes conflicting emotions. Humans also respond to these emotions in complex ways, such that conscious goals, values, and other cognitions influence behavior in addition to emotional responses.
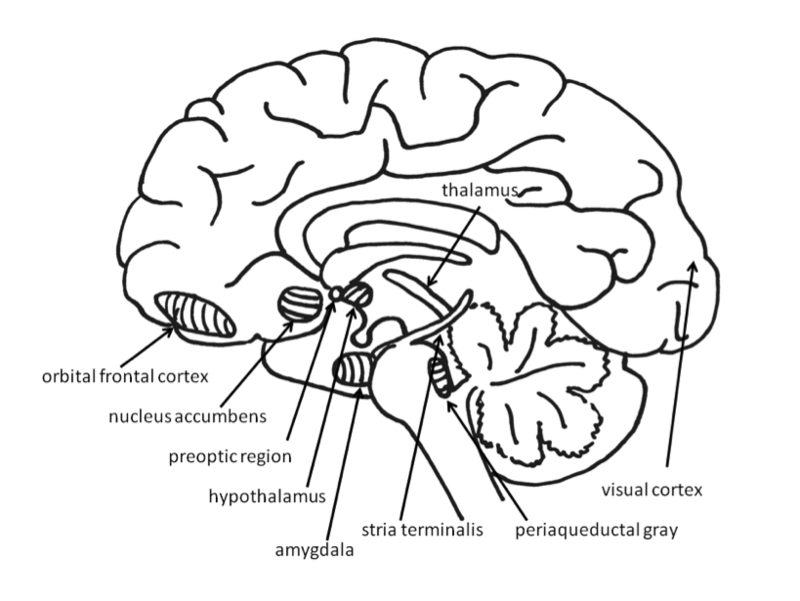
Across species, emotional responses are organized around the organism’s survival and reproductive needs. Emotions influence perception, cognition, and behavior to help organisms survive and thrive (Farb, Chapman, & Anderson, 2013). Networks of structures in the brain respond to different needs, with some overlap between different emotions. Specific emotions are not located in a single structure of the brain. Instead, emotional responses involve networks of activation, with many parts of the brain activated during any emotional process. In fact, the brain circuits involved in emotional reactions include nearly the entire brain (Berridge & Kringelbach, 2013). Brain circuits located deep within the brain below the cerebral cortex are primarily responsible for generating basic emotions (Berridge & Kringelbach, 2013; Panksepp & Biven, 2012). In the past, research attention was focused on specific brain structures that will be reviewed here, but future research may find that additional areas of the brain are also important in these processes.
Basic Emotions
Desire: The neural systems of reward seeking
One of the most important affective neuronal systems relates to feelings of desire, or the appetite for rewards. Researchers refer to these appetitive processes using terms such as “wanting” (Berridge & Kringelbach, 2008), “seeking” (Panksepp & Biven, 2012), or “behavioural activation sensitivity” (Gray, 1987). When the appetitive system is aroused, the organism shows enthusiasm, interest, and curiosity. These neural circuits motivate the animal to move through its environment in search of rewards such as appetizing foods, attractive sex partners, and other pleasurable stimuli. When the appetitive system is underaroused, the organism appears depressed and helpless.
Much evidence for the structures involved in this system comes from animal research using direct brain stimulation. When an electrode is implanted in the lateral hypothalamus or in cortical or mesencephalic regions to which the hypothalamus is connected, animals will press a lever to deliver electrical stimulation, suggesting that they find the stimulation pleasurable. The regions in the desire system also include the amygdala, nucleus accumbens, and frontal cortex (Panksepp & Biven, 2012). The neurotransmitter dopamine, produced in the mesolimbic and mesocortical dopamine circuits, activates these regions. It creates a sense of excitement, meaningfulness, and anticipation. These structures are also sensitive to drugs such as cocaine and amphetamines because they are dopamine agonists (Panksepp & Biven, 2012).

Research in both humans and nonhuman animals shows that the left frontal cortex (compared to the right frontal cortex) is more active during appetitive emotions such as desire and interest. Researchers first noted that persons who had suffered damage to the left frontal cortex developed depression, whereas those with damage to the right frontal cortex developed mania (Goldstein, 1939). The relationship between left frontal activation and approach-related emotions (that is those emotions that involve a movement toward the stimulus such as with happiness and also with anger) has been confirmed in healthy individuals using EEG and fMRI (Berkman & Lieberman, 2010). For example, increased left frontal activation occurs in 2- to 3-day-old infants when sucrose is placed on their tongues (Fox & Davidson, 1986), and in hungry adults as they view pictures of desirable desserts (Gable & Harmon-Jones, 2008). In addition, greater left frontal activity in appetitive situations has been found to relate to dopamine (Wacker, Mueller, Pizzagalli, Hennig, & Stemmler, 2013).
“Liking”: The Neural Circuits of Pleasure and Enjoyment
Surprisingly, the amount of desire an individual feels toward a reward need not correspond to how much he or she likes that reward. This is because the neural structures involved in the enjoyment of rewards are different from the structures involved in the desire for the rewards. “Liking” (e.g., enjoyment of a sweet liquid) can be measured in babies and nonhuman animals by measuring licking speed, tongue protrusions, and happy facial expressions, whereas “wanting” (desire) is shown by the willingness to work hard to obtain a reward (Berridge & Kringelbach, 2008). Liking has been distinguished from wanting in research on topics such as drug abuse. For example, drug addicts often desire drugs even when they know that the ones available will not provide pleasure (Stewart, de Wit, & Eikelboom, 1984).
Research on liking has focused on a small area within the nucleus accumbens and on the posterior half of the ventral pallidum. These brain regions are sensitive to opioids and endocannabinoids (endogenously produced substances that have effects similar to marijuana). Stimulation of other regions of the reward system increases wanting, but does not increase liking, and in some cases even decreases liking. The research on the distinction between desire and enjoyment contributes to the understanding of human addiction, particularly why individuals often continue to frantically pursue rewards such as cocaine, opiates, gambling, or sex, even when they no longer experience pleasure from obtaining these rewards due to habituation.
The experience of pleasure also involves the orbitofrontal cortex. Neurons in this region fire when monkeys taste, or merely see pictures of, desirable foods. In humans, this region is activated by pleasant stimuli including money, pleasant smells, and attractive faces (Gottfried, O’Doherty & Dolan, 2002; O’Doherty, Deichmann, Critchley, & Dolan, 2002; O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; O’Doherty, Winston, Critchley, Perrett, Burt, & Dolan, 2003).
Fear: The Neural System of Freezing and Fleeing

Fear is an unpleasant emotion that motivates avoidance of potentially harmful situations. Slight stimulation of the fear-related areas in the brain causes animals to freeze, whereas intense stimulation causes them to flee. The fear circuit extends from the central amygdala to the periaqueductal gray in the midbrain. These structures are sensitive to glutamate, corticotrophin releasing factor, adreno-cortico-trophic hormone, cholecystokinin, and several different neuropeptides. Benzodiazepines and other tranquilizers inhibit activation in these areas (Panksepp & Biven, 2012). As is discussed in a later section, stress plays a role in releasing these chemicals activating the fight/flight/freeze response. As discussed elsewhere (Chapter 6 and again in Chapter 16), benzodiazepines are used to lower anxiety or anticipation of a fearful situation by inhibiting the activity of these areas because they are GABA agonists.
The role of the amygdala in fear responses has been extensively studied. Perhaps because fear is so important to survival, two pathways send signals to the amygdala from the sensory organs. When an individual sees a snake, for example, the sensory information travels from the eye to the thalamus and then to the visual cortex. The visual cortex sends the information on to the amygdala, provoking a fear response. However, the thalamus also quickly sends the information straight to the amygdala, so that the organism can react before consciously perceiving the snake (LeDoux, Farb, & Ruggiero, 1990). The pathway from the thalamus to the amygdala is fast but less accurate than the slower pathway from the visual cortex. Damage to the amygdala or areas of the ventral hippocampus interferes with fear conditioning in both humans and nonhuman animals (LeDoux, 1996).
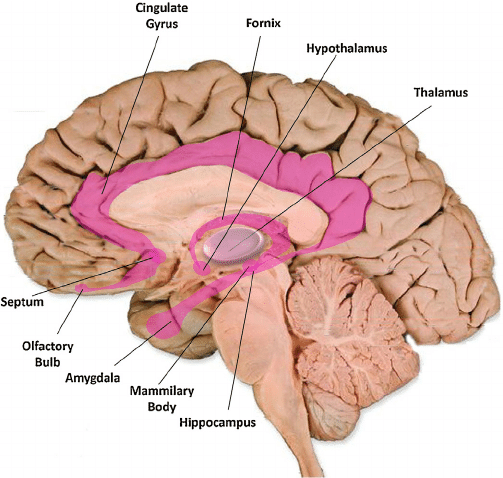
Love: The Neural Systems of Care and Attachment

For social animals such as humans, attachment to other members of the same species produces the positive emotions of attachment: love, warm feelings, and affection. The emotions that motivate nurturing behavior (e.g., maternal care) are distinguishable from those that motivate staying close to an attachment figure in order to receive care and protection (e.g., infant attachment). Important regions for maternal nurturing include the dorsal preoptic area (Numan & Insel, 2003) (see Figure ) and the bed nucleus of the stria terminalis(Panksepp, 1998).
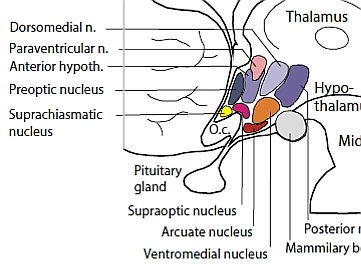
These regions overlap with the areas involved in sexual desire, and are sensitive to some of the same neurotransmitters, including oxytocin, arginine-vasopressin, and endogenous opioids (endorphins and enkephalins).
Grief: The Neural Networks of Loneliness and Panic
The neural networks involved in infant attachment are also sensitive to separation. These regions produce the painful emotions of grief, panic, and loneliness. When infant humans or other infant mammals are separated from their mothers, they produce distress vocalizations, or crying. The attachment circuits are those that cause organisms to produce distress vocalizations when electrically stimulated.
The attachment system begins in the midbrain periaqueductal gray, very close to the area that produces physical pain responses, suggesting that it may have originated from the pain circuits (Panksepp, 1998). Separation distress can also be evoked by stimulating the dorsomedial thalamus, ventral septum, dorsal preoptic region, and areas in the bed nucleus of stria terminalis (near sexual and maternal circuits). (Panksepp, Normansell, Herman, Bishop, & Crepeau, 1988)
These regions are sensitive to endogenous opiates, oxytocin, and prolactin. All of these neurotransmitters prevent separation distress. Opiate drugs such as morphine and heroin, as well as nicotine, artificially produce feelings of pleasure and gratification, similar to those normally produced during positive social interactions. This may explain why these drugs are addictive. Panic attacks appear to be an intense form of separation distress triggered by the attachment system, and panic can be effectively relieved by opiates. Testosterone also reduces separation distress, perhaps by reducing attachment needs. Consistent with this, panic attacks are more common in women than in men.
Knowledge Emotions
Paul Silvia (University of North Carolina, Greensboro) suggested that when people think of emotions they usually think of the obvious ones, such as happiness, fear, anger, and sadness. He instead looks at the knowledge emotions, a family of emotional states that foster learning, exploring, and reflecting. Surprise, interest, confusion, and awe come from events that are unexpected, complicated, and mentally challenging, and they motivate learning in its broadest sense, be it learning over the course of seconds (finding the source of a loud crash, as in surprise) or over a lifetime (engaging with hobbies, pastimes, and intellectual pursuits, as in interest). Of course there are several causes, consequences, and individual differences. As a group, the knowledge emotions motivate people to engage with new and puzzling things rather than avoid them. Over time, engaging with new things, ideas, and people broadens someone’s experiences and cultivates expertise. The knowledge emotions thus don’t gear up the body like fear, anger, and happiness do, but they do gear up the mind—a critical task for humans, who must learn essentially everything that they know.
So while emotions are something we think of in terms of feelings and the fight or flight response, or even as longer lasting sadness or love, Silvia's ideas point out the less noticed strong connection between emotion and cognition. It is clear that emotions can and do provide a strong basis for how and what we think about.
Plasticity: Experiences Can Alter the Brain
The responses of specific neural regions may be modified by experience. For example, the front shell of the nucleus accumbens is generally involved in appetitive behaviors, such as eating, and the back shell is generally involved in fearful defensive behaviors (Reynolds & Berridge, 2001, 2002). Research using human neuroimaging has also revealed this front–back distinction in the functions of the nucleus accumbens (Seymour, Daw, Dayan, Singer, & Dolan, 2007). However, when rats are exposed to stressful environments, their fear-generating regions expand toward the front, filling almost 90% of the nucleus accumbens shell. On the other hand, when rats are exposed to preferred home environments, their fear-generating regions shrink and the appetitive regions expand toward the back, filling approximately 90% of the shell (Reynolds & Berridge, 2008). Consider how this might be generalized to human experiences that are stressful versus those that are generally comforting and comfortable.
Brain Structures Have Multiple Functions
Although much affective neuroscience research has emphasized whole structures, such as the amygdala and nucleus accumbens, it is important to note that many of these structures are more accurately referred to as complexes. They include distinct groups of nuclei that perform different tasks. At present, human neuroimaging techniques such as fMRI are unable to examine the activity of individual nuclei in the way that invasive animal neuroscience can. For instance, the amygdala of the nonhuman primate can be divided into 13 nuclei and cortical areas (Freese & Amaral, 2009). These regions of the amygdala perform different functions. The central nucleus sends outputs involving brainstem areas that result in innate emotional expressions and associated physiological responses. The basal nucleus is connected with striatal areas that are involved with actions such as running toward safety. Furthermore, it is not possible to make one-to-one maps of emotions onto brain regions. For example, extensive research has examined the involvement of the amygdala in fear, but research has also shown that the amygdala is active during uncertainty (Whalen, 1998) as well as positive emotions (Anderson et al., 2003; Schulkin, 1990).
Conclusion
Research in affective neuroscience has contributed to knowledge regarding emotional, motivational, and behavioral processes. The study of the basic emotional systems of nonhuman animals provides information about the organization and development of more complex human emotions. Although much still remains to be discovered, current findings in affective neuroscience have already influenced our understanding of drug use and abuse, psychological disorders such as panic disorder, and complex human emotions such as desire and enjoyment, grief and love.
Outside Resources
- Video: A 1-hour interview with Jaak Panksepp, the father of affective neuroscience
- Video: A 15-minute interview with Kent Berridge on pleasure in the brain
- Video: A 5-minute interview with Joseph LeDoux on the amygdala and fear
- Web: Brain anatomy interactive 3D model
References
- Anderson, A. K., Christoff, K., Stappen, I., Panitz, D., Ghahremani, D. G., Glover, G., . . . Sobel, N. (2003). Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience, 6, 196–202.
- Berkman, E. T., & Lieberman, M. D. (2010). Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience, 22(9), 1970–1979. doi: 10.1162/jocn.2009.21317
- Berridge, K. C., & Kringelbach, M. L. (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. Current Opinion in Neurobiology, 23, 294–303. doi.org/10.1016/j.conb.2013.01.017
- Berridge, K. C., & Kringelbach, M. L. (2008). Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology, 199, 457–480. doi: 10.1007/s00213-008-1099-6
- Blanchard, D. C., & Blanchard, R. J. (2003). What can animal aggression research tell us about human aggression? Hormones and Behavior, 44, 171–177.
- Farb, N.A.S., Chapman, H. A., & Anderson, A. K. (2013). Emotions: Form follows function. Current Opinion in Neurobiology, 23, 393–398. http://dx.doi.org/10.1016/j.conb.2013.01.015
- Fox, N. A., & Davidson, R. J. (1986). Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia, 24, 417–422.
- Freese, J. L., & Amaral, D. G. (2009). Neuroanatomy of the primate amygdala. In P. J. Whalen & E. A. Phelps (Eds.), The human amygdala (pp. 3–42). New York, NY: Guilford Press.
- Gable, P. A., & Harmon-Jones, E. (2008). Relative left frontal activation to appetitive stimuli: Considering the role of individual differences. Psychophysiology, 45, 275-278.
- Goldstein, K. (1939). The organism: An holistic approach to biology, derived from pathological data in man. New York, NY: American Book.
- Gottfried, J. A., O’Doherty, J., & Dolan, R. J. (2002). Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. Journal of Neuroscience, 22, 10829–10837.
- Gray, J. A. (1987). The psychology of fear and stress (2nd ed.). Cambridge, England: Cambridge University Press.
- Harmon-Jones, E., Harmon-Jones, C., & Price, T. F. (2013). What is approach motivation? Emotion Review, 5, 291–295. doi: 10.1177/1754073913477509
- Heinrichs, M., von Dawans, B., & Domes, G. (2009). Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology, 30, 548–557.
- Izard, C. E. (2010). The many meanings/aspects of emotion: Definitions, functions, activation, and regulation. Emotion Review, 2, 363–370. doi: 10.1177/1754073910374661
- LeDoux, J. E. (1996). The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Simon & Schuster.
- LeDoux, J. E., Farb, C. F., Ruggiero, D. A. (1990). Topographic organization of neurons in the acoustic thalamus that project to the amygdala. Journal of Neuroscience, 10, 1043–1054.
- Numan, M., & Insel, T. R. (2003). The neurobiology of parental behavior. New York, NY: SpringerVerlag.
- O’Doherty J. P., Deichmann, R., Critchley, H. D., & Dolan, R. J. (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33, 815–826.
- O’Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J., & Andrews, C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4, 95–102.
- O’Doherty, J., Winston, J., Critchley, H., Perrett, D., Burt, D. M., & Dolan, R. J. (2003). Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia, 41, 147–155.
- Panksepp, J. (1998). Affective neuroscience: The foundations of human and animal emotions. New York, NY: Oxford University Press.
- Panksepp, J., & Biven, L. (2012). The archaeology of mind: Neuroevolutionary origins of human emotions. New York, NY: Norton.
- Panksepp, J., Normansell, L., Herman, B., Bishop, P., & Crepeau, L. (1988). Neural and neurochemical control of the separation distress call. In J. D. Newman (Ed.), The physiological control of mammalian vocalization (pp. 263–299). New York, NY: Plenum.
- Peterson, C. K., & Harmon-Jones, E. (2012). Anger and testosterone: Evidence that situationally-induced anger relates to situationally-induced testosterone. Emotion, 12, 899–902. doi: 10.1037/a0025300
- Reynolds, S. M., & Berridge, K. C. (2008). Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience, 11, 423–425.
- Reynolds, S. M., & Berridge, K. C. (2002). Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience, 22, 7308–7320.
- Reynolds, S. M., & Berridge, K. C. (2001). Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. Journal of Neuroscience, 21, 3261–3270.
- Schulkin, J. (1991). Sodium hunger: The search for a salty taste. New York, NY: Cambridge University Press.
- Seymour, B., Daw, N., Dayan, P., Singer, T., & Dolan, R. (2007). Differential encoding of losses and gains in the human striatum. Journal of Neuroscience, 27, 4826–4831.
- Stewart, J., De Wit, H., & Eikelboom, R. (1984). Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review, 91, 251-268.
- Wacker, J., Mueller, E. M., Pizzagalli, D. A., Hennig, J., & Stemmler, G. (2013). Dopamine-D2-receptor blockade reverses the association between trait approach motivation and frontal asymmetry in an approach-motivation context. Psychological Science, 24(4), 489–497. doi: 10.1177/0956797612458935
- Whalen, P. J. (1998). Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7, 177–188.
Attributions:
Affective Neuroscience By Eddie Harmon-Jones and Cindy Harmon-Jones, University of New South Wales, Noba Project, licensed CC BY-NC-SA 4.0
File:BrainCaudatePutamen.svg: User:LeevanjacksonDerivative work: User:SUM1, CC BY-SA 4.0, via Wikimedia Commons
Images are generated by Life Science Databases(LSDB)., CC BY-SA 2.1 JP, via Wikimedia Commons
Sanador2.0, CC BY-SA 3.0, via Wikimedia Commons
Knowledge Emotions by Paul Silvia, University North Carolina, Greensboro, Noba Project, licensed CC BY-NC-SA 4.0


