16.3: Fight or Flight
- Page ID
- 130348
This page is a draft and under active development. Please forward any questions, comments, and/or feedback to the ASCCC OERI (oeri@asccc.org).
- Compare different theories about the generation of emotions.
- Describe the anatomical and chemical bases of anger and fear.
- Analyze how epigenetic research has enhanced our understanding of the nature-nurture debate surrounding anger and fear.
Overview
An important function of emotions in humans, and other animals that live in groups of any kind, is to communicate with other members of one's own species. The emotions that are particularly important to communicate to other members are those related to fear, and those related to anger. When we think of the physiological arousal associated with emotions, we often talk about the "fight or flight" response. These correspond nicely with the two major negative emotions - anger and fear. Multiple animal paradigm studies and human neuroscientific research, including studies with psychopathological conditions, have served to examine the nature of these emotions. Some of these theories and findings will be discussed.
Theories of Emotional Distinctions
Can emotions be better described as qualitatively distinct, for example, as discrete “basic emotions” or “natural kinds” (Ekman et al., 1983; Izard, 1992; Panksepp, 2005) or as quantitatively distinct, for example, as points along a circumplex defined by dimensions like arousal and valence (Russell and Barrett, 1999; Barrett and Wager, 2006)? Recent years have seen a protracted debate in the literature about how to most accurately capture the nature of emotion (Barrett et al., 2007; Izard, 2007; Panksepp, 2007; Tracy and Randles, 2011), with proposed models of emotion including not only basic emotion and dimensional models, but also those that focus upon goal-relevant appraisals of emotional stimuli (Moors et al., 2013), emotions as coping responses (Roseman, 2013), and emotions as survival circuits (LeDoux, 2012). An extended conversation about the strengths and weaknesses of these various views will not be reviewed in full here, rather, the focus will be on the basic consideration of whether different emotions (e.g., fear, anger) are best viewed as qualitatively or quantitatively distinct.
Qualitative Ideas
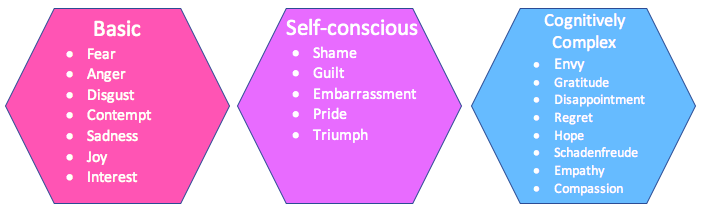
Models that posit emotions to be qualitatively distinct, such as “basic emotion” models, holds that a limited number of emotions like fear, anger, and positive excitement emerge from dissociable neurophysiological processes (Ekman et al., 1983; Izard, 1992; Panksepp, 2005; Lench et al., 2011). In Figure \(\PageIndex{1}\) we see one representation of this idea that initially we have basic emotions including fear, anger, disgust, contempt, joy, sadness and interest, which then grow to include self conscious emotions (guilt, pride, embarrassment, shame, triumph) and finally cognitively complex emotions (envy, gratitude, disappointment, regret, hope, schadenfreude, empathy, compassion). These neurophysiological processes are generally linked to activity in the evolutionarily ancient subcortical structures of the midbrain, striatum, and limbic system most commonly linked to emotion (Panksepp, 2005; Vytal and Hamann, 2010). So, for example, the generation of positive excitement is linked to activation in a striatal circuit centered on dopaminergic neurons in the nucleus accumbens (Ikemoto and Panksepp, 1999), whereas the generation of fear is associated with activity in a circuit involving the periaqueductal gray, anterior and medial hypothalamus, and amygdala (LeDoux, 2000). In this view, finer gradations of experience result when basic emotions are modulated or elaborated by higher-level cognitive processes controlled by the cerebral cortex, but the emergence of qualitatively distinct emotions is not dependent on these cortically-controlled processes (Panksepp, 2005).
Quantitative Ideas
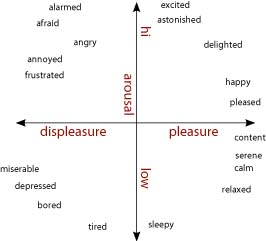
Models that posit emotions to be quantitatively distinct hold that emotions like fear, anger, and happiness are best described as points on one or more core dimensions. Core dimensions typically proposed to distinguish among emotions are physiological arousal or activation (low—high) and valence (bad—good) (Bradley et al., 2001). [Some have proposed a withdrawal—approach dimension as a substitute or supplement to the valence axis (Wager et al., 2003; Christie and Friedman, 2004; van Honk and Schutter, 2006)]. As shown in figure \(\PageIndex{2}\), arranged orthogonally, these dimensions form a circumplex upon which emotions can be plotted and quantitatively compared (Barrett and Russell, 1999; Russell and Barrett, 1999; Colibazzi et al., 2010). Positive excitement is plotted as high in arousal and positive in valence, and sadness is low in arousal and negative in valence. Fear is typically plotted as high arousal and strongly negative, as is anger (Russell and Barrett, 1999). Further distinctions among emotions are thought to reflect differences in cognitive construals of the events surrounding the basic changes in arousal and valence. Thus, whether an individual experiences anger or fear (which are similar in terms of arousal or valence) may be shaped by interpretations of neurophysiological changes in valence and arousal in light of the eliciting stimulus and the individual's idiosyncratic stores of semantic knowledge, memories, and behavioral responses that shape the subjectively experienced state (Russell, 2003). Under this view, distinctions among experienced emotional states are highly dependent on these cognitively complex processes, which are subserved by a distributed network of regions of the cerebral cortex (Lindquist et al., 2012).
These models generate distinct predictions to the question of whether a disorder or lesion could result in a single emotion being disabled without affecting the experience of other emotions. The discrete emotions view would argue that a disorder or lesion that resulted in dysfunction in the specific structures subserving a particular emotion could affect the experience of one emotion while leaving others intact. In contrast, the dimensional view would require either that other emotions that are dimensionally similar to the affected emotion also be affected, or that deficits in a particular emotion would reflect dysfunction in cortically-driven higher-level cognitive processes.
Psychopathy Supports the Qualitative Model
The case of psychopathy lends clear support to notion that fear is qualitatively distinct from other emotions. In psychopathy, the bulk of the clinical and empirical evidence points toward the conclusion that fear responding is uniquely disabled, with other high-arousal (positive excitement, anger) and negatively valenced (anger, disgust) emotions remaining intact. The dimensional view cannot easily explain why in psychopaths the high arousal, negatively valenced state of anger is easily (perhaps too easily) generated, whereas the high arousal, negatively valenced state of fear is not. The problem cannot lie in a failure to fully engage neurocognitive systems underlying either the arousal or valence dimension, because psychopaths experience other high-arousal emotions (positive excitement) as well as other negatively valenced emotions (disgust). It also cannot result from some difficulty arising at the interaction of these axes, because anger and fear are highly similar in terms of both dimensions. Models that substitute a withdrawal—approach axis for a negative—positive axis are no more successful; the two most strongly withdrawal-linked emotions are disgust and fear, and there is no evidence for disgust-based impairments in psychopathy. Individuals with psychopathy also fail to recognize and therefore have no empathic response to others’ fear.
On the whole, the empirical data support the idea that the amygdala, along with its efferent projections, is an essential structure for the generation of conditioned fear responses, which account for the majority of experienced fear (Davis, 1992, 1997). Extensive early evidence demonstrated that the amygdala plays a crucial role in the creation of conditioned fear in rodents. For example, lesions to the amygdala prevent rats from developing a conditioned fear response, like freezing in response to a stimulus that predicts shock (Blanchard and Blanchard, 1972). Later studies clarified the roles of the various subnuclei of the amygdala, demonstrating that the lateral nucleus is primarily involved in the acquisition of the fear response whereas the central nucleus is involved in both the acquisition and the expression of conditioned fear responses (Davis, 1992; Wilensky et al., 2006). The amygdala's many efferent projections coordinate autonomic and behavioral responses to fear eliciting stimuli. Projections from the central nucleus of the amygdala to the lateral hypothalamus are involved in activating autonomic sympathetic nervous system responses, and projections to the ventrolateral periaqueductal gray direct the expression of behavior responses, such as defensive freezing (Davis, 1992; LeDoux, 2012). The amygdala's central role in coordinated fear responding can be demonstrated by electrical stimulation studies showing that complex patterns of behavioral and autonomic changes associated with fear responses result from stimulation of the relevant regions of the amygdala (Davis, 1992). Heavy reliance on animal models is justified in the study of fear responding and the amygdala given how strongly conserved the amygdala nuclei involved in responding to conditioned threats are across species ranging from reptiles to birds to rodents to primates (LeDoux, 2012).
Research Suggesting Connections Between Anger and Fear
Neumann et al. (2010) hypothesize that aggressive behaviors, that are of two basic types - reactive and proactive, are mediated by anxiety-based neurological bases. Based on animal models, they suggest the following: "Male aggression is necessary for the acquisition and maintenance of nutrition, territory, and mating partners. Species-specific rules have to be strictly obeyed to guarantee effective and harmless communication. Thus, adaptive offensive aggression is comprised primarily of harmless threat behaviors allowing the opponent to escape or to switch to submissive behaviors in order to avoid direct physical confrontation. In rodents, such signs of offensive aggression include piloerection (intimidation of the opponent by larger appearance) and lateral threat (arched back and exposure of the flank). In case of an offensive attack, less vulnerable body parts of the opponent, such as those covered with muscles and a thick layer of skin, are targeted to avoid serious injuries (Blanchard and Blanchard, 1977 ; Blanchard et al., 2003 ). While offensive aggression is usually expressed during a fight for territory or exclusive mating, defensive aggression is mainly displayed in life-threatening situations and is linked to increased fear (Blanchard and Blanchard, 1981 ). As opposed to offensive aggression, defensive aggression is less or not signaled in advance, and attack targets include more vulnerable body parts (such as the head, belly, and genitals) (Blanchard and Blanchard, 1977 ; Blanchard et al., 2003 )." The resident-intruder animal paradigms have been used to measure these ideas (see Figure \(\PageIndex{3}\)).

They also claim: "Anxiety may be interpreted as an emotional anticipation of an aversive situation and is reflected by species-specific behavioural fear responses to stressful and threatening stimuli characteristic for individual trait anxiety. Fear is not seen as basal state (as is anxiety), but as a complex behavioural response, such as startle or freezing. Further, in addition to factors which determine innate (trait) anxiety, several environmental or pharmacological factors may interact with the genetic background and determine the individual level of state anxiety and the final behavioural phenotype. Emotionality, often used as synonym for anxiety as well as fearfulness, may be seen in a broader sense, comprising both trait and state anxiety and stimulus-related fear. Emotionality is one of the major components underlying the ability of an organism to assess stressful stimuli and scenarios, and to adequately cope with them." (Neumann et al., 2010)
Neumann et al. (2010) show that several clinical and laboratory studies with humans and rodents have shown that there are complicated mechanisms by which anxiety and aggression are co-regulated. There are several neurochemicals found to be involved in complex ways as follows:
Glucocorticoids - The regulation of the HPA (Hypothalamic-Pituitary-Adrenal) axis seems very much connected to the experience of anxiety and aggression. However, the direction is not straightforward in that both high and low glucocorticoids are related to high aggression.
Vasopressin - Vasopressin is produced in the hypothalamus, and testosterone is produced in the gonads, and both modulate aggression as well as anxiety in males. Vasopressin is also involved in reducing anxiety as well as pair-bonding.
Testosterone - Along with aggression, testosterone rises in puberty, castration reduces both (through reduction in Vasopressin also). There are some environmental factors that have a moderating effect, but in general, testosterone is clearly related to modulating aggression in males.
Serotonin - lower 5-HT seems linked to greater aggression and violence in female rhesus. Genetic factors and early life stressors tend to mediate these effects.
GABA - when anxiety levels are increased, aggression increased depending on genetic predispositions to it, and activity also increased in "brain regions including the central amygdala, BST (Bed nucleus of the stria terminalis), lateral septum, and PVN (paraventricular nucleus) that are associated with stress-, fear- and aggression-related behaviour."
"The striking evidence for an overlap in neuroendocrine and neurochemical systems regulating aggression as well as anxiety suggests a strong correlation between these two behaviours. Thus, aggression and anxiety are not always co-regulated, but, under some circumstances, these behaviours may come under the control of the same genes and neuroactive substances including sexual steroids, neuropeptides and neuroamines within specific brain circuitries. Such a view is in agreement with clinical findings. On the one hand, excessive and violent behaviours are seen in humans exposed to adverse early life experiences and in patients with depression- and anxiety-related disorders, or PTSD. On the other hand, rather conflicting data exist on the effects of anxiolytic drugs on anti-social and aggressive behaviours. In future studies that focus on the neurobiological mechanisms of (co-)regulation of aggression and anxiety, epigenetic modifications need to be considered in addition to the neuronal and neuroendocrine parameters discussed above." (Neumann et al, 2010).
Nature v. Nurture
Both genetic factors and experiences play a role in aggressive behaviors. The genetic regulation of serotonin release appears to play a role in aggression in many mammals including humans. For example, Peeters et al.'s (2020) study concluded that the short version of the serotonin transporter gene (S-allele of the 5-HTTLPR) seems to be linked to greater reactive aggression, and those individuals with higher avoidance tendencies toward angry facial expressions. Their findings indicate that evaluative impulses in response to social cues play an important role in mediating the genetic predisposition of the 5-HTTLPR polymorphism to increased expression of reactive aggression. Because of the chief role of MAOs in the metabolism of key neurotransmitters involved in aggressive behavior, most notably serotonin, it is not surprising that a substantial body of research has linked aggressive phenotypes with the MAO system. Many results inspired both the scientific community and media outlets to refer to MAO-A as the warrior or criminal gene. Although initial pharmacological studies supported a role for MAOIs in the reduction of aggressive phenotypes, data from these studies were hard to interpret because of the side effects of MAOIs, and because of their impact on a myriad of unrelated behaviors. Substantive evidence supporting the role of the MAO genes in aggression comes from studies in knockout (KO) mice. Selective knockout models for the MAO-A gene, for instance, exhibited increased aggressiveness compared to their wild-type counterparts. Genetic studies in humans provide evidence that MAO-A is linked to aggression but only when other environmental factors are also present during development (e.g., abuse, stressors). Other lines of research have demonstrated that the gene product of MAO-A (rather than the gene per se) influences violent traits. For instance, cortical and subcortical MAO-A activity in vivo -measured with positron emission tomography (PET)- was negatively associated with trait aggression (attribution: Mentis, Dardiotis, Katsouni & Chrousos, 2021).
Over the last two decades, the study of the relationship between nature and nurture in shaping human behavior has encountered a renewed interest. Behavioral genetics showed that distinct polymorphisms of genes that code for proteins that control neurotransmitter metabolic and synaptic function are associated with individual vulnerability to aversive experiences, such as stressful and traumatic life events, and may result in an increased risk of developing psychopathologies associated with violence. On the other hand, recent studies indicate that experiencing aversive events modulates gene expression by introducing stable changes to DNA without modifying its sequence, a mechanism known as “epigenetics”. For example, experiencing adversities during periods of maximal sensitivity to the environment, such as prenatal life, infancy and early adolescence, may introduce lasting epigenetic marks in genes that affect maturational processes in brain, thus favoring the emergence of dysfunctional behaviors, including exaggerate aggression in adulthood.
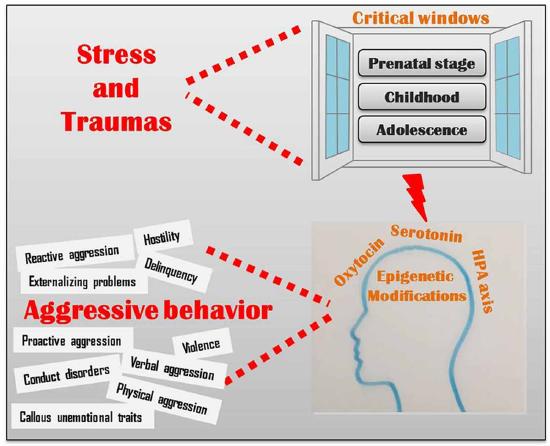
Adverse environmental influences during critical periods of development have been correlated with epigenetic markers that affect glucocorticoid receptor function. As seen in many studies, low cortisol release is correlated with low self control and higher aggression. Production of oxytocin is related to higher social functioning and attachment formation. Oxytocin secretion is stimulated by early maternal care, whereas adverse experiences in prenatal and early infancy development are related to lower oxytocin receptor methylation (a process involved in epigenetics). Serotonin is related to regulation of aggression. Genes responsible for controlling the reuptake of serotonin from the synaptic cleft are affected by early stressors (including trauma as well as conditions generated by poverty like poor medical care, housing quality and exposure to violent neighborhoods) that alter brain anatomy and function such as cortical thickness and amygdala reactivity. (See Figure \(\PageIndex{4}\) for the multiple ways in which stress and traumas lead to epigenetic modifications in the oxytocin, serotonin and HPA axis systems that affect aggressive behavior: reactive and proactive aggression, hostility, delinquency externalizing problems, violence, conduct disorders, physical and verbal aggression and callous unemotional traits.)
Palumbo, Mariotti, Ioffreda and Pellegrini (2018) conclude that epigenetics is shedding a new light on the fine interaction between nature and nurture, by providing a novel tool to understand the molecular events that underlie the relationship among genes, brain, environment and behavior. Altogether, the results of the studies that we briefly discussed in the present article, clearly indicate that, when it comes to (human) behavior, nature and nurture are not to be regarded as two distinct and separate factors, contrary to the alternating predominance of either one that has been proposed in different historic phases (Levitt, 2013; Moore, 2016). Indeed, distinct genetic backgrounds differentially modulate the individual susceptibility to the environment and at the same time various environmental conditions differentially affect gene expression, in an intimate and fascinating manner that scientists have now begun to disentangle. The findings from this research pave the way to a novel approach to the understanding of human behavior, with important implications also for social sciences, including philosophy, ethics and law.
Conclusions
Anger and fear are considered basic emotions. While they are primarily negative, they serve very important survival functions. They appear to have clear genetic and biological bases that have been discussed in the research above.
References
- Barrett, L. F., Lindquist, K. A., Bliss-Moreau, E., Duncan, S., Gendron, M., Mize, J., et al. (2007). Of mice and men: natural kinds of emotions in the mammalian brain? A response to Panksepp and Izard. Perspect. Psychol. Sci. 2, 297–312. doi: 10.1126/science.7652558
- Barrett, L. F., and Russell, J. A. (1999). The structure of current affect. Curr. Dir. Psychol. Sci. 8, 10.
- Barrett, L. F., and Wager, T. D. (2006). The structure of emotion: Evidence from neuroimaging studies. Curr. Dir. Psychol. Sci. 5, 79–83.
- Blanchard, D. C., and Blanchard, R. J. (1972). Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psychol. 81, 281–290.
- Bradley, M. M., Codispoti, M., Cuthbert, B. N., and Lang, P. J. (2001). Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion 1, 276–298. https://doi.org/10.1037/1528-3542.1.3.276
- Buckholtz, J. W. & Meyer-Lindenberg, A. (2008). MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. https://doi.org/10.1016/j.tins.2007.12.006
- Cases, O. et al. (1995). Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science (80-) https://doi.org/10.1126/science.7792602
- Christie, I. C., and Friedman, B. H. (2004). Autonomic specificity of discrete emotion and dimensions of affective space: a multivariate approach. Int. J. Psychophysiol. 51, 143–153. https://doi.org/10.1016/j.ijpsycho.2003.08.002
- Colibazzi, T., Posner, J., Wang, Z., Gorman, D., Gerber, A., Yu, S., et al. (2010). Neural systems subserving valence and arousal during the experience of induced emotions. Emotion 10, 377–389. https://doi.org/10.1037/a0018484
- Davis, M. (1992). The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 15, 353–375. doi/10.1146/annurev.ne.15.030192.002033
- Davis, M. (1997). Neurobiology of fear responses: the role of the amygdala. J. Neuropsychiatry Clin. Neurosci. 9, 382–402. https://doi.org/10.1176/jnp.9.3.382
- Ekman, P., Levenson, R. W., and Friesen, W. V. (1983). Autonomic nervous system activity distinguishes among emotions. Science 221, 1208–1210. doi: 10.1126/science.6612338
- Feinstein, J. S., Buzza, C., Hurlemann, R., Follmer, R. L., Dahdaleh, N. S., Coryell, W. H., et al. (2013). Fear and panic in humans with bilateral amygdala damage. Nat. Neurosci. 16, 270–222. https://doi.org/10.1016/j.cub.2010.11.042
- Ficks, C. A. & Waldman, I. D. (2014). Candidate genes for aggression and antisocial behavior: a meta-analysis of association studies of the 5HTTLPR and MAOA-uVNTR. Behav. Genet. https://doi.org/10.1007/s10519-014-9661-y
- Godar, S. C., Fite, P. J., McFarlin, K. M. & Bortolato, M. (2016). The role of monoamine oxidase A in aggression: current translational developments and future challenges. Prog. Neuro-Psychopharmacol. Biol. Psychiatry https://doi.org/10.1016/j.pnpbp.2016.01.001
- Ikemoto, S., and Panksepp, J. (1999). The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 31, 6–41. https://doi.org/10.1016/S0165-0173(99)00023-5
- Izard, C. E. (1992). Basic emotions, relations among emotions, and emotion-cognition relations. Psychol. Rev. 99, 561–565. https://doi.org/10.1037/0033-295X.99.3.561
- Izard, C. E. (2007). Basic emotions, natural kinds, emotion schemas, and a new paradigm. Perspect. Psychol. Sci. 2, 260–280.
- Johnson, P. L., Fitz, S. D., Hollis, J. H., Moratalla, R., Lightman, S. L., Shekhar, A., et al. (2011). Induction of c-Fos in “panic/defence”-related brain circuits following brief hypercarbic gas exposure. J. Psychopharmacol. 25, 26–36. https://doi.org/10.1177/0269881109353464
- LeDoux, J. E. (2000). Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184. https://doi.org/10.1146/annurev.neuro.23.1.155
- LeDoux, J. (2012). Rethinking the emotional brain. Neuron 73, 653–676. https://doi.org/10.1016/j.neuron.2012.02.004
- Lench, H. C., Flores, S. A., and Bench, S. W. (2011). Discrete emotions predict changes in cognition, judgment, experience, behavior, and physiology: a meta-analysis of experimental emotion elicitations. Psychol. Bull. 137, 834–855. https://doi.org/10.1037/a0024244
- Levitt, M. (2013). Perceptions of nature, nurture and behaviour. Life Sci. Soc. Policy, 9(13). doi: 10.1186/2195-7819-9-13
- Lindquist, K. A., Wager, T. D., Kober, H., Bliss-Moreau, E., and Barrett, L. F. (2012). The brain basis of emotion: a meta-analytic review. Behav. Brain. Sci. 35, 121–143. doi:10.1017/S0140525X11000446
- Moore, D. S. (2016). Behavioral epigenetics. Wiley Interdiscip. Rev. Syst. Biol. Med. 9:e1333. doi: 10.1002/wsbm.1333
- Moors, A., Ellsworth, P. C., Scherer, K. R., and Frijda, N. H. (2013). Appraisal theories of emotion: State of the art and future development. Emot. Rev. 5, 119–124.
- Panksepp, J. (2005). Affective consciousness: core emotional feelings in animals and humans. Conscious. Cogn. 14, 30–80. https://doi.org/10.1016/j.concog.2004.10.004
- Neumann I.D., Veenema, A.H. & Beiderbeck, D.I. (2010) Aggression and anxiety: social context and neurogiological links. Front. Behav. Neurosci. 4(12). doi: 10.3389/fnbeh.2010.00012
- Panksepp, J. (2007). Neurologizing the psychology of affects. Perspect. Psychol. Sci. 2, 281–296.
- Peeters, D. G. A., Lange, W.-G., von Borries A. K. L., Franke, B., Volman I., Homberg J. R., Verkes R.-J., Roelofs, K. (2020). Threat-avoidance tendencies moderate the link between serotonin transporter genetic variation and reactive aggression. Frontiers in Behavioral Neuroscience, 14, 172. doi:10.3389/fnbeh.2020.562098
- Popova, N. K. (2006). From genes to aggressive behavior: the role of serotonergic system. BioEssays https://doi.org/10.1002/bies.20412
- Roseman, I. (2013). Appraisal in the emotion system: coherence in strategies for coping. Emot. Rev. 5, 141–149.
- Ruisch, I. H., Dietrich, A., Glennon, J. C., Buitelaar, J. K. & Hoekstra, P. J. (2019). Interplay between genome-wide implicated genetic variants and environmental factors related to childhood antisocial behavior in the UK ALSPAC cohort. Eur. Arch. Psychiatry Clin. Neurosci. https://doi.org/10.1007/s00406-018-0964-5
- Russell, J. A. (2003). Core affect and the psychological construction of emotion. Psychol. Rev. 110, 145–172. https://doi.org/10.1037/0033-295X.110.1.145
- Russell, J. A., and Barrett, L. F. (1999). Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Pers. Soc. Psychol. 76, 805–819. https://doi.org/10.1037/0022-3514.76.5.805
- Shih, J. C. & Chen, K. (1999). MAO-A and -B gene knock-out mice exhibit distinctly different behavior. Neurobiology, 7, 235–246 (1999).
- Sohrabi, S. (2015). The criminal gene: the link between MAOA and aggression (REVIEW). BMC Proc. https://doi.org/10.1186/1753-6561-9-s1-a49
- Takahashi, A., Quadros, I. M., de Almeida, R. M. M. & Miczek, K. A. (2012). Behavioral and pharmacogenetics of aggressive behavior. Curr. Top. Behav. Neurosci. https://doi.org/10.1007/7854_2011_191 Sohrabi, S. (2015). The criminal gene: the link between MAOA and aggression (REVIEW). BMC Proc. https://doi.org/10.1186/1753-6561-9-s1-a49
- Takahashi, A., Quadros, I. M., de Almeida, R. M. M. & Miczek, K. A. (2012). Behavioral and pharmacogenetics of aggressive behavior. Curr. Top. Behav. Neurosci. https://doi.org/10.1007/7854_2011_191
- Tracy, J. L., and Randles, D. (2011). Four models of basic emotions: a review of Ekman and Cordaro, Izard, Levenson, and Panksepp and Watt. Emot. Rev. 3, 397–405.
- van Honk, J., and Schutter, D. J. L. G. (2006). From affective valence to motivational direction The frontal asymmetry of emotion revised. Psychol. Sci. 17, 963–965. https://doi.org/10.1111/j.1467-9280.2006.01813.x
- Vassos, E., Collier, D. A. & Fazel, S. (2014). Systematic meta-analyses and field synopsis of genetic association studies of violence and aggression. Mol. Psychiatry https://doi.org/10.1038/mp.2013.31
- Vytal, K., and Hamann, S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 22, 2864–2885. https://doi.org/10.1162/jocn.2009.21366
- Wager, T. D., Phan, K. L., Liberzon, I., and Taylor, S. F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19, 513–531. https://doi.org/10.1016/S1053-8119(03)00078-8
- Waltes, R., Chiocchetti, A. G. & Freitag, C. M. (2016). The neurobiological basis of human aggression: a review on genetic and epigenetic mechanisms. Am. J. Med. Genet. Part B Neuropsychiatr.Genet. https://doi.org/10.1002/ajmg.b.32388
- Wilensky, A. E., Schafe, G. E., Kristensen, M. P., and LeDoux, J. E. (2006). Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 26, 12387–12396. https://doi.org/10.1523/JNEUROSCI.4316-06.2006
- Youdim, M. B. H., Edmondson, D. & Tipton, K. F. (2006). The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. https://doi.org/10.1038/nrn1883
Attributions
What can we learn about emotions by studying psychopathy by Abigail Marsh, in Frontiers in Human Neuroscience licensed CC BY 3.0
Psychologist Russell's model of arousal and valence by http://imagine-it.org/gamessurvey/, licensed CC BY 3.0, via Wikimedia Commons
Individual emotions by U3161650, CC BY-SA 4.0, via Wikimedia Commons
Image by DataBase Center for Life Science (DBCLS) licensed CC-BY 4.0 via Wikimedia commons.
From warrior genes to translational solutions: novel insights into monoamine oxidases (MAOs) and aggression. by Alexios-Fotios A. Mentis, Efthemios Dardiotis, Eleni Katsouni, George P. Chrousos. in Transl Psychiatry licensed CC-BY 4.0
Genes and Aggressive Behavior: Epigenetic Mechanisms Underlying Individual Susceptibility to Aversive Environments by Sara Palumbo, Veronica Mariotti, Caterina Ioffreda & Silvia Pellegrini in Frontiers in Behavioral Neuroscience (2018) licensed CC BY 4.0


