9.4: Synaptic Mechanisms of Learning and Memory
- Last updated
- Save as PDF
- Page ID
- 217210

- Kenneth A. Koenigshofer
- Cosumnes River College
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Describe Hebb's theory of the engram
- Define Hebb's rule and Hebb synapses
- Describe the synaptic changes mediating habituation and sensitization in the sea slug
- Explain the dual-trace theory of memory
- Describe the role of the hippocampus in learning and memory
- Describe the role of the cerebellum, basal ganglia, and amygdala in learning and memory
- Explain the concepts and associated neural changes of Long-term Potentiation (LTP) and Long-term depression
Overview
Donald Hebb's (1949) dual-trace theory of the physical basis of memory focused on changes at the synapse as the basis for learning and memory. This influential theory led to the discovery of changes in transmitter release associated with learning in the invertebrate nervous system of the sea slug. Other researchers found sustained increases in synaptic strength caused by high frequency stimulation of the pre-synaptic neuron (Long-term Potentiation, LTP) as well as sustained decrease in synaptic strength known as Long-term Depression (LTD). Researchers have also discovered anatomical changes in dendritic spines associated with LTP and learning and memory, suggesting anatomical changes at synapses as a possible mechanism for learning and long-term memory. Although early studies in search of the "engram", the physical basis for learning and memory, concluded that these functions were widely distributed throughout the brain, later research found that the hippocampus is critical for formation of long-term explicit memories and that the cerebellum is involved in implicit memory, while the amygdala plays an essential role in emotional memories.
The Search for Learning and Memory in the Synapse
Just as information is stored digitally in "the cloud" or on hard drives and flash drives, the information in our long-term memory must be physically stored in the brain. According to current theory, the ability to maintain information in long-term memory involves a gradual strengthening of the synaptic connections among neurons. When pathways in these neural networks are frequently and repeatedly activated, the synapses become more efficient permitting enhanced communication among neurons in the network, and these changes create memory (Saylor Foundation, 2012). As we will see, this view of the physical basis of memory was heavily influenced by the ideas of a Harvard biological psychologist named Donald Hebb.
Over a century ago a Russian physiologist named Ivan Pavlov proposed a theory of how learning occurred in the brain. As you recall, Pavlov discovered classical conditioning when he observed that repeated pairings of a bell and food eventually led dogs to salivate at the sound of the bell, even when food was not present. To explain this phenomenon, Pavlov hypothesized that the conditioned stimulus (CS), a bell in this case, generated a locus of neural activity in sensory cortex (auditory cortex in this experiment) which radiated outward over the cortical surface. This was followed by a similar locus of cortical neural activity generated by the unconditioned stimulus (US) (meat powder in Pavlov's classic experiments) which also set up waves of radiating neural activity. Pavlov proposed that these two expanding fields of neural activity, originating from different areas of cortex, would intersect one another. According to Pavlov, this intersection of the cortical fields of neural activity, generated by the CS and US, formed the neurological basis for the association between CS and US.
This early theory of the physical basis of learning and memory was put to the test by experimental psychologist, Karl Lashley in the mid 20th century. Lashley made cuts throughout the cerebral cortex of rats and then attempted to condition them. He did this to disrupt any radiating neural activity in the cortex that might be present during conditioning, as Pavlov had proposed. Lashley found that the rats still could be conditioned and that they retained the conditioned response later in spite of the crisscross cuts over their entire cerebral cortex. This disproved Pavlov's theory that the physical basis of learning an association by classical conditioning was the intersection of waves of neural activity radiating outward from cortical loci.
Lashley, like Pavlov, was interested in finding the "engram." This term refers to the physical memory trace, the neural representation of memory in the brain. Toward this end, Lashley performed additional experiments in which he trained rats to navigate a maze and then destroyed different parts of their brains. He found that no matter where the brain damage was located, rats still retained some memory of the maze (Lashley, 1929, 1943, 1950). Lashley interpreted these results as evidence that it was the amount of cortical tissue removed, not its location, that determined the degree of impairment in learning and memory. He also hypothesized that memories were widely distributed throughout the brain and that therefore there was no particular area of the brain that was especially critical for memory formation.
Lashley proposed two principles derived from his research on the physical basis of learning and memory: 1) mass action (the amount of cortex destroyed determines degree of impairment in learning and memory) and 2) equipotentiality (any part of cortex within a functional area can take over the functions of any other part of that same functional area).
Lashley's conclusions had two primary impacts. First, his analysis "tended to discredit, or at least deemphasize, the role of the interior [subcortical] parts of the brain in learning and memory and, on the other, tended to localize the mechanisms of learning and memory within the confines of the cerebral cortex" (Thompson, 1974). Second, Lashley's principles of mass action and equipotentiality led to an anti-localization bias in the study of learning and memory, which discouraged attempts to find specific brain structures involved in these functions. However, consistent with current views, and contradicting Lashley's findings, Kaada, Rasmussen, and Kveim (1961) reported that lesions of the hippocampo-fornix system impaired maze performance. This was an early study that confirms what we know today, that a subcortical structure, the hippocampus, is critical to many forms of learning and memory.
Hebb's Rule, Cell Assemblies, and the Engram
After receiving his doctorate from Harvard in 1936, one of Lashley's graduate students, Donald Hebb, published The Organization of Behavior: A Neuropsychological Theory. In this influential book, Hebb proposed that the "engram" consisted of changes at synapses during learning and memory formation. A quote from the book (Hebb, 1949, p. 62) explains Hebb's central principle of synaptic change in learning and memory:
“When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased."
Here Hebb is proposing an increase in the strength of synaptic connections between neurons that consistently fire together and that this involves growth or metabolic events in one or both of the cells.
More simply stated: "Neurons that fire together, wire together." This is known as "Hebb's rule" (retrieved from https://can-acn.org/donald-olding-hebb/ August 8, 2021).
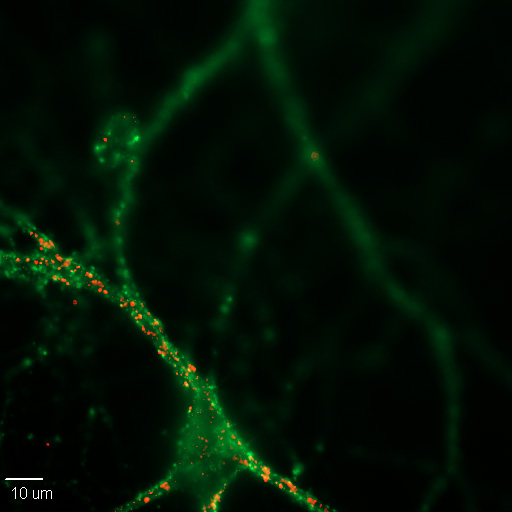
Figure \(\PageIndex{1}\): A 20X image of a cultured mouse cortical neuron in cell culture. Synapses are labeled for pre- (green) and post-synaptic (red) proteins, synaptophysin and PSD-95, respectively. (Image and caption from Wikimedia, Synapse; https://commons.wikimedia.org/wiki/F...n_Synapses.jpg; author, Dchordpdx; licensed under the Creative Commons Attribution 4.0 International license).
Neuroscientists use the term, Hebbian synapses, to refer to synapses that follow this principle. Hebb also proposed the idea of "cell assemblies." Hebb hypothesized that when cells repeatedly firing together, wired together, eventually they would form larger structures, "cell assemblies," which would form neural representations of whole, complex perceptions, ideas, memories, and other cognitive structures, such as schemas, and highly abstract categories and concepts. All of this could be built from changes in the efficiency of synaptic transmission at specific synapses, following Hebb's principle that "neurons that fire together, wire together." One can imagine that entire cell assemblies that fire together might also wire together, creating much larger and much more complex neural representations in the brain of complex, abstract ideas including such things as scientific theories and mathematical formulations.
Non-Association Learning in Aplysia
These ideas have inspired the hunt by neuroscientists for changes at synapses and in pre- or post-synaptic neurons, during and after learning--changes which might be the physical basis in the nervous system for learning and memory (Lashley's "engram," which, by the way, has nothing to do with the non-scientific use of the term by Scientology). Much of the pioneering scientific work on the search for the "engram" has involved looking for these physical changes associated with learning in the simple nervous system of a marine species, Aplysia californica, a type of sea slug. A major reason for the selection of this species for study, aside from the simplicity of its nervous system (just 20,000 neurons), is the large size of its neurons which makes the work and the observations easier.

Figure \(\PageIndex{2}\): Aplysia californica. (Image from Wikimedia, Aplysia californica; https://commons.wikimedia.org/wiki/F...,_Monterey.jpg; Chad King / NOAA MBNMS; this image is in the public domain because it contains materials that originally came from the U.S. National Oceanic and Atmospheric Administration, taken or made as part of an employee's official duties).
Eric Kandel (1976) and his colleagues did pioneering work which showed synaptic changes that mediate habituation (see sections above) of the Aplysia's siphon and gill defensive withdrawal reflex. A decrease in conductivity was found at synapses involved in the siphon and gill withdrawal reflex in Aplysia after habituation to a repeated presentation of a harmless novel stimulus. Kandel and co-workers showed that the pre-synaptic sensory neuron released less neurotransmitter onto the post-synaptic motor neuron as a result of repeated presentation of the novel stimulus leading to habituation of the reflex. The opposite was found for increased responsiveness.
Sensitization refers to enhancement of responsiveness to a familiar stimulus. A single small electric shock to the tail of Aplysia heightens its gill withdrawal response for minutes to hours. Kandel and colleagues found that sensitization was mediated by an increase in release of neurotransmitter from the pre-synaptic sensory neuron onto the post-synaptic motor neuron serving the gill muscle. Thus, sensitization is the opposite of habituation both behaviorally and at the level of transmitter release (Kolb and Whishaw, 2001). Later studies showed synaptic changes involving modified transmitter release during classical conditioning as well (Kandel and Schwartz, 1982).
The Special Roles of the Hippocampus, Cerebellum, and Amygdala
As discussed above, research since Lashley has revealed that his speculation that all areas of brain were equally involved in learning and memory was incorrect. Now we know that one of the most important brain regions in explicit memory is the hippocampus, which serves as a preprocessor and elaborator of information (Squire, 1992). The hippocampus helps us encode information about spatial relationships, the context in which events were experienced, and the associations among memories (Eichenbaum, 1999). The hippocampus also serves in part as a switching point that holds the memory for a short time and then directs the information to other parts of the brain, such as the cortex, to actually do the rehearsing, elaboration, and long-term storage (Jonides, Lacey, & Nee, 2005; Saylor Foundation, 2015). We also now know that different parts of the brain are involved in different kinds of memory.
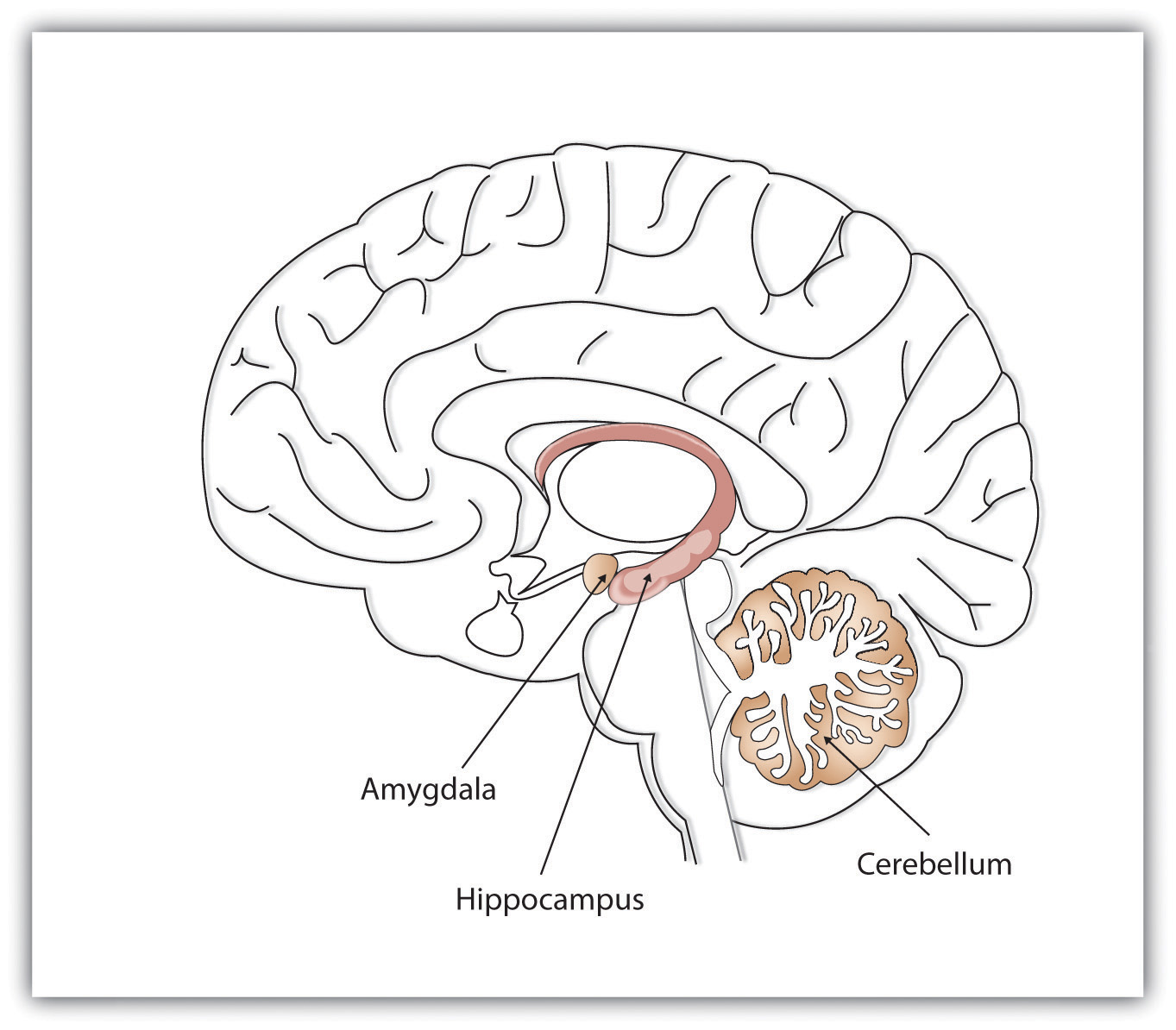
Figure \(\PageIndex{3}\): Different brain structures help us remember different types of information. The hippocampus is particularly important in explicit memories, the cerebellum in implicit memories, and the amygdala in emotional memories (Image and caption from the Saylor Foundation, 2015; Remembering and Judging; https://learn.umgc.edu/d2l/le/conten.../25917628/View; licensed under a Creative Commons Attribution 3.0 Unported License.).
While the hippocampus is handling explicit memory, the cerebellum and the amygdala are critically involved in implicit and emotional memories, respectively. Research shows that the cerebellum is more active when we are learning associations and in priming tasks (based on implicit memory), and animals and humans with damage to the cerebellum are impaired in classical conditioning (Krupa, Thompson, & Thompson, 1993; Woodruff-Pak, Goldenberg, Downey-Lamb, Boyko, & Lemieux, 2000). The storage of many of our most important emotional memories, and particularly those related to fear, is initiated and controlled by the amygdala (Sigurdsson, Doyère, Cain, & LeDoux, 2007).
Changes at the Synapse Correlated with Learning and Memory
Hebb's theory that long-term memory was stored by physical changes at the synapse has been so influential that research on the neural basis of learning and memory has focused primarily on synaptic events. Focus on the synapse was reinforced by the discovery in 1973 in rabbit hippocampus that a long-lasting increase in synaptic conductivity (synaptic strength) could be produced by high frequency stimulation of the pre-synaptic neuron (Bliss and Lomo, 1973). This finding that synaptic strength can be increased for an extended period of time following high frequency pre-synaptic stimulation is now referred to as Long-Term Potentiation (LTP). LTP has been found in many species and in many parts of the brain. However, it has been studied most in the hippocampus of the rat. Since this early research, additional forms of synaptic change have also been discovered.
Synaptic Plasticity
Synaptic plasticity is the strengthening or weakening of synapses over time in response to increases or decreases in their activity. Plastic change also results from the alteration of the number of receptors located at a synapse. Synaptic plasticity is the basis of learning and memory, enabling a flexible, functioning nervous system. Synaptic plasticity can be either short-term (synaptic enhancement or synaptic depression) or long-term. Two processes in particular, long-term potentiation (LTP) and long-term depression (LTD), are important forms of synaptic plasticity that occur in synapses in the hippocampus.
Long-term Potentiation (LTP)
Long-term potentiation (LTP) is a persistent strengthening of a synaptic connection, which can last for minutes or hours or even weeks. LTP is based on the Hebbian principle: “cells that fire together wire together.” There are various mechanisms, none of which are fully understood, behind the synaptic strengthening seen with LTP.
One known mechanism involves a type of postsynaptic glutamate receptor: NMDA (N-Methyl-D-aspartate) receptors. These receptors are normally blocked by magnesium ions. However, when the postsynaptic neuron is depolarized by multiple presynaptic inputs in quick succession (either from one neuron or multiple neurons), the magnesium ions are forced out and Ca2+ ions pass into the postsynaptic cell. Next, Ca2+ ions entering the cell initiate a signaling cascade that causes a different type of glutamate receptor, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, to be inserted into the postsynaptic membrane. Activated AMPA receptors allow positive ions to enter the cell.
Therefore, the next time glutamate is released from the presynaptic membrane, it will have a larger excitatory effect (EPSP) on the postsynaptic cell because the binding of glutamate to these AMPA receptors will allow more positive ions into the cell. The insertion of additional AMPA receptors strengthens the synapse so that the postsynaptic neuron is more likely to fire in response to presynaptic neurotransmitter release.
LTP has many similarities with the synaptic changes Hebb proposed as basis for long-term memory, including two key features. First, LTP is long lasting, up to a year with repeated trials. Second, many forms of LTP require simultaneous activation in pre-synaptic and post-synaptic neurons at the synapse where LTP takes place ("neurons that fire together, wire together"); this is because NMDA (N-methyl-d-aspartate) receptors for glutamate (the most common excitatory transmitter in the brain), which are prominent at synapses where LTP occurs, have the same requirement for simultaneous activity in both pre- and post-synaptic neurons in order for these receptors to become activated. Research showing that many features of LTP are similar to features of long-term memory provides strong circumstantial evidence that LTP is related to the mechanisms of learning and memory. For example, LTP can be stimulated by low intensity stimulation similar to that produced in single neurons; LTP is most prominent in structures associated with learning; LTP is produced in the hippocampus by learning; drugs that enhance or impair learning also enhance or impair LTP; and LTP occurs in the nervous system of simple invertebrates (see discussion of Aplysia above) at the specific synapses involved in the learning (Pinel & Barnes, 2021).
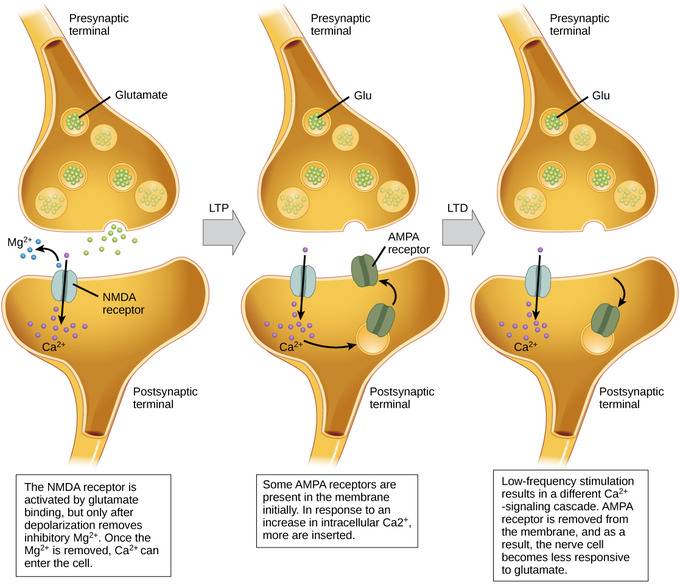
Figure \(\PageIndex{4}\): Long-term potentiation and depression: Calcium entry through postsynaptic NMDA receptors can initiate two different forms of synaptic plasticity: long-term potentiation (LTP) and long-term depression (LTD). LTP arises when a single synapse is repeatedly stimulated. This stimulation causes a calcium- and CaMKII-dependent cellular cascade, which results in the insertion of more AMPA receptors into the postsynaptic membrane. The next time glutamate is released from the presynaptic cell, it will bind to both NMDA and the newly-inserted AMPA receptors, thus depolarizing the membrane more efficiently. LTD occurs when few glutamate molecules bind to NMDA receptors at a synapse (due to a low firing rate of the presynaptic neuron). The calcium that does flow through NMDA receptors initiates a different calcineurin and protein phosphatase 1-dependent cascade, which results in the endocytosis of AMPA receptors. This makes the postsynaptic neuron less responsive to glutamate released from the presynaptic neuron. (Image and caption from Lumen Boundless Biology, How Neurons Communicate; https://courses.lumenlearning.com/bo...s-communicate/; curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike.)
Long-term Depression (LTD)
Another related phenomenon is long-term depression (LTD), associated with decreases in synaptic conductivity. LTD might be part of the processes involved in creating and modifying patterns of excitation and inhibition in large populations of neurons for the coding of learned movements, sensory experience, and perhaps the mental representations of complex cognitive structures such as perceptions, whole memories, concepts, and even abstract ideas (Churchland, 2013). A related possibility is the pruning away of some synapses to form permanent changes in neural circuitry. Long-term depression (LTD) is essentially the reverse of LTP: it is a long-term weakening of a synaptic connection. One mechanism known to cause LTD also involves AMPA receptors. In this situation, calcium that enters through NMDA receptors initiates a different signaling cascade, which results in the removal of AMPA receptors from the postsynaptic membrane. With the decrease in AMPA receptors in the membrane, the postsynaptic neuron is less responsive to the glutamate released from the presynaptic neuron. While it may seem counterintuitive, LTD may be just as important for learning and memory as LTP. The weakening and pruning of unused synapses trims unimportant connections, leaving only the salient connections strengthened by long-term potentiation.
Changes at the Synapse Correlated with Learning and Memory: Anatomical Changes in Dendritic Spines
Modifications in synaptic strengths in excitatory synapses in the hippocampus appear to play a critical role in storage and recall of information in mammals. In humans at least, the role of the hippocampus is especially critical in explicit episodic (autobiographical) memory. As noted above, it is also crucially involved in spatial memory for many mammal species, including humans. Changes in synaptic strength throughout much of the brain may be important in learning and memory.
Dendritic Spines
Dendritic spines are tiny protruding structures located on the shaft of dendrites and are associated with synaptic connections. These spines "are present in large numbers on the surface of dendrites. For example, a single pyramidal neuron in the hippocampal CA1 region possesses as many as 30,000 dendritic spines. A majority of excitatory synapses are formed on the surface of these dendritic spines" (Irie & Yamaguchi, 2009, p. 1141). The primary sites of excitatory synaptic interaction in the mammalian central nervous system appear to be at dendritic spines.
Changes in spines play a significant role during brain development. "In a developing brain, spines exhibit a high degree of structural and functional plasticity, reflecting the formation and elimination of synapses during the maturation of neuronal circuits. The morphology of spines in developing neurons is affected by synaptic activity, hence contributing to the experience-dependent refinement of neuronal circuits, learning, and memory. Thus, understanding spine dynamics and its regulation is of central importance to studies of synaptic plasticity in the brain" (Bertling, et al., 2012, p. 391). During brain development synapses are formed, modified and sometimes eliminated as a function of input to them.
As discussed above, LTP has been shown to lead to functional changes at synapses where it is induced. These changes due to LTP are increased synaptic conductivity and enhanced responsiveness of the post-synaptic neuron. It appears that anatomical changes in spines accompany these changes in synaptic strength which occur during LTP and learning and memory. Changes in synaptic strength, associated with LTP, are accompanied by LTP-induced alterations of the shape and size of dendritic spines (Chidambaram, et al., 2019; Harris, et al., 2003). This is in line with the anatomical changes at the synapse that Hebb predicted.
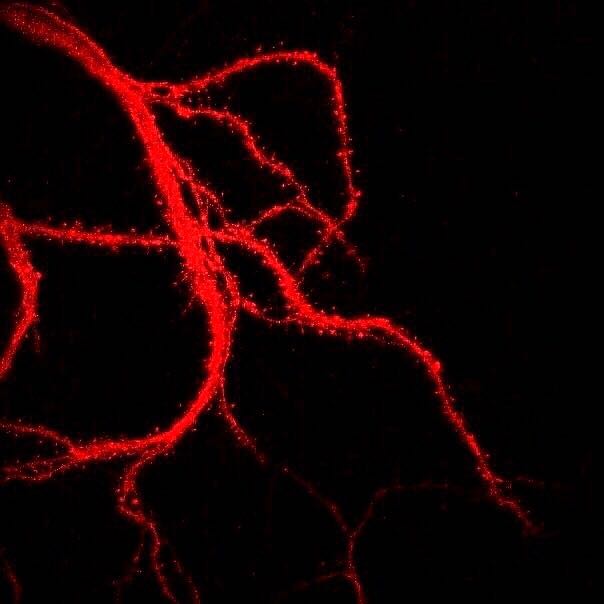
Figure \(\PageIndex{5}\): Branching dendrites of a neuron showing dendritic spines (tiny bristle-like projections lining each dendritic branch). The photograph was obtained with a laser scanning microscope. Dendritic spines can rapidly change in size and shape and numbers, and are important in learning and memory. (Caption by Kenneth A. Koenigshofer, PhD. Image from Wikimedia Commons; File:Нейрональные отростки с шипиками.jpg; https://commons.wikimedia.org/wiki/F...0%BC%D0%B8.jpg; by Sergb95; licensed under the Creative Commons Attribution-Share Alike 4.0 International license).
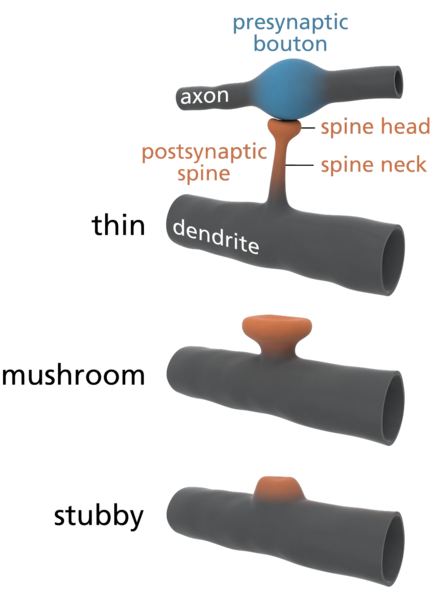
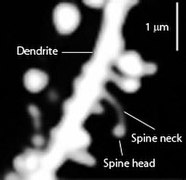
Figure \(\PageIndex{6}\): (Left) Varying morphology of types of dendritic spines. Changes in their shape and size are associated with LTP and learning and memory. (Right) Spines on the dendrite of a medium spiny striatal neuron. The image was obtained by expressing Enhanced Green Fluorescent Protein (EGFP) in the neurons and imaging them using a laser scanning two photon microscope. (Caption for image on left by Kenneth A. Koenigshofer, PhD; Image on left from Wikimedia Commons, Dendritic spines; https://commons.wikimedia.org/wiki/F...e_types_3D.png; original work of Thomas Splettstoesser (www.scistyle.com); licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. Image on right and its caption from Wikimedia Commons, Dendritic spines; https://commons.wikimedia.org/w/inde...=Go&type=image; Released into Public Domain by the image author).
Chidambaram, et al. (2019, p.161) summarize the relationship of changes in synaptic conductivity to changes in dendritic spines: "During synaptic plasticity the number and shapes of dendritic spines undergo radical reorganizations. Long-term potentiation (LTP) induction promotes spine head enlargement and the formation and stabilization of new spines. Long-term depression (LTD) results in their shrinkage and retraction."
Hebb's theory has proven to be impressively predictive of discoveries made many years after his book was published in 1949. Findings like those described in this module continue to confirm many aspects of Hebb's theory.
Additional Mechanisms of LTP
“Silent synapses” are another mechanism that was discovered in the mid-1990s and that may contribute to long-term potentiation (LTP). These synapses are physically present, but under normal conditions do not contribute to synaptic transmission.
Some of these silent synapses have been found in the hippocampus. They appear to have receptors for NMDA but not for AMPA. It is thought that these synapses may be activated during LTP and thus help to strengthen the synaptic response. The discovery that after LTP, these synapses do display an electrical current associated with AMPA channels suggests that some newly synthesized AMPA receptors may be inserted into the post-synaptic membrane.
In addition to all of the post-synaptic mechanisms involved in the establishment of LTP, it has long been postulated that some pre-synaptic modifications occur during the ensuing maintenance phase. But certain modifications, such as an increase in the amount of glutamate released by the pre-synaptic neuron, would imply the presence of a retrograde messenger that goes back to this neuron and modifies it. Because nitric oxide (NO) is a gas in its natural state, and can thus diffuse through cell membranes, it would be an ideal candidate for this role. But its involvement is still the subject of much debate and controversy.
The following videos provide a useful animated review of the key elements of LTP:
References
Bennett E.L., et al. (1964). Chemical and anatomical plasticity of the brain. Science, 146:610. [PubMed]
Bertling, E., Ludwig, A., Koskinen, M., & Hotulainen, P. (2012). Methods for three-dimensional analysis of dendritic spine dynamics. In Methods in enzymology (Vol. 506, pp. 391-406). Academic Press.
Bliss T.V. & Collingridge G.L. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 361 (6407): 31–39
Bliss, T. V., Collingridge, G. L., Morris, R. G., & Reymann, K. G. (2018). Long-term potentiation in the hippocampus: discovery, mechanisms and function. Neuroforum, 24 (3), A103-A120.
Cherubini, E., & Miles, R. M. (2015). The CA3 region of the hippocampus: how is it? What is it for? How does it do it?. Frontiers in cellular neuroscience, 9, 19.
Citri A., & Malenka R.C. (2008). Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 33 (1): 18–41.
Colley P.A., Routtenberg A. (1993). Long-term potentiation as synaptic dialogue. Brain Res Brain Res Rev. 18:115. [PubMed]
DeZazzo J., Tully T. (1995). Dissection of memory formation: from behavioral pharmacology to molecular genetics. Trends Neurosci. 18:212. [PubMed]
Hebb, D.O. (1949). The Organization of Behavior. New York. Wiley.
Irie, F., & Yamaguchi, Y. (2009). Eph receptor signaling and spine morphology. Encyclopedia of Neuroscience, p. 1141-1145.
Koskinen, M., Bertling, E., & Hotulainen, P. (2012). Methods to measure actin treadmilling rate in dendritic spines. In Methods in enzymology (Vol. 505, pp. 47-58). Academic Press.
Krasne F.B., Glanzman D.L. (1995). What we can learn from invertebrate learning. Annu Rev Psychol. 46:585.
Leuner, B., & Shors, T. J. (2010). Synapse formation and memory. In Encyclopedia of Behavioral Neuroscience (pp. 349-355). Elsevier Inc.
Mizumori S.J., Rosenzweig M.R., Bennett E.L. (1985). Long-term working memory in the rat: effects of hippocampally applied anisomycin. Behav Neurosci.;99:220. [PubMed]
Nicoll, R. A., & Kauer, J. A. Malenka. RC 1988. The current excitement in long-term potentiation. Neuron, 1, 97-103.
Park, P., Georgiou, J., Sanderson, T.M. et al. (2021). PKA drives an increase in AMPA receptor unitary conductance during LTP in the hippocampus. Nat Commun 12, 413. This article is licensed under a Creative Commons Attribution 4.0.
Rose S.P.R. (1992). Neuropsychology of Memory. Squire L.R., et al., editors. Guilford; New York: p. 547.
Rose S.P. (1995). Glycoproteins and memory formation. Behav Brain Res. 66:73. [PubMed]
Rosenzweig M.R., et al. (1992). Neuropsychology of Memory. Squire LR, et al., editors. Guilford; New York: p. 533.
Rosenzweig, M. R. (2007). Modification of brain circuits through experience. In Neural Plasticity and Memory: From Genes to Brain Imaging. CRC Press/Taylor & Francis, Boca Raton (FL); 2007. PMID: 21204433.
Serrano P.A., et al. (1994). Differential effects of protein kinase inhibitors and activators on memory formation in the 2-day-old chick. Behav Neural Biol. 61:60. [PubMed]
Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine, R. (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62 (3): 405–496
Tully T, et al. A return to genetic dissection of memory in Drosophila. Cold Spring Harb Symp Quant Biol. 1996;61:207. [PubMed]
Yang, S., Yang, S., Moreira, T., Hoffman, G., Carlson, G. C., Bender, K. J., ... & Tang, C. M. (2014). Interlamellar CA1 network in the hippocampus. Proceedings of the National Academy of Sciences, 111(35), 12919-12924.
Additional References
Alvarez, V. A., & Sabatini, B. L. (2007). Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci., 30, 79-97.
Bliss, T. V., & Lømo, T. (1973). Long‐lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of physiology, 232(2), 331-356.
Chidambaram, S. B., Rathipriya, A. G., Bolla, S. R., Bhat, A., Ray, B., Mahalakshmi, A. M., ... & Sakharkar, M. K. (2019). Dendritic spines: revisiting the physiological role. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 92, 161-193.
Eichenbaum, H. (1999). Conscious awareness, memory, and the hippocampus. Nature Neuroscience, 2 (9), 775–776.
Hayashi-Takagi, A., Yagishita, S., Nakamura, M., Shirai, F., Wu, Y.I., Loshbaugh, A.L., Kuhlman, B., Hahn, KM., Kasai, H. (2015). Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525, 333–338.
Harris, K. M., Fiala, J. C., & Ostroff, L. (2003). Structural changes at dendritic spine synapses during long-term potentiation. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358 (1432), 745-748.
Hebb, D.O. (1949). The Organization of Behavior. New York. Wiley.
Henry, F. E., Hockeimer, W., Chen, A., Mysore, S. P., & Sutton, M. A. (2017). Mechanistic target of rapamycin is necessary for changes in dendritic spine morphology associated with long-term potentiation. Molecular Brain, 10 (1), 1-17.
Jonides, J., Lacey, S. C., & Nee, D. E. (2005). Processes of working memory in mind and brain. Current Directions in Psychological Science, 14 (1), 2–5.
Kaada, B. R., Rasmussen, E. W., & Kveim, O. (1961). Effects of hippocampal lesions on maze learning and retention in rats. Experimental Neurology, 3(4), 333-355.
Kandel, E. (1976). Cellular Basis of Behavior. San Francisco. W.H. Freeman and Company.
Kandel, E. R., & Schwartz, J. H. (1982). Molecular biology of learning: Modulation of transmitter release. Science, 218 (4571), 433–443
Kasai, Haruo; Matsuzaki, Masanori; Noguchi, Jun; Yasumatsu, Nobuaki; Nakahara, Hiroyuki (1 July 2003). "Structure–stability–function relationships of dendritic spines". Trends in Neurosciences. 26 (7): 360–368.
Kasthuri, N., Hayworth, K. J., Berger, D. R., Schalek, R. L., Conchello, J. A., Knowles-Barley, S., ... & Lichtman, J. W. (2015). Saturated reconstruction of a volume of neocortex. Cell, 162 (3), 648-661.
Kim, C. H., & Lisman, J. E. (1999). A role of actin filament in synaptic transmission and long-term potentiation. Journal of Neuroscience, 19 (11), 4314-4324.
Kolb, B. & Whishaw, I.Q. (2001). In Introduction to Brain and Behavior. New York. Worth Publishers.
Krucker, T., Siggins, G. R., & Halpain, S. (2000). Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proceedings of the National Academy of Sciences, 97(12), 6856-6861.
Krupa, D. J., Thompson, J. K., & Thompson, R. F. (1993). Localization of a memory trace in the mammalian brain. Science, 260(5110), 989–991.
Lashley, K. S. (1929). The effects of cerebral lesions subsequent to the formation of the maze habit: Localization of the habit. In Brain mechanisms and intelligence: A quantitative study of injuries to the brain (pp. 86–108). Chicago, IL: University of Chicago Press.
Miller, R. R., & Marlin, N. A. (2014). Amnesia following electroconvulsive shock. Functional disorders of memory, 143-178. New York. Psychology Press.
Murakoshi, H., Wang, H., & Yasuda, R. (2011). Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature, 472 (7341), 100-104.
Ofer, N., Berger, D. R., Kasthuri, N., Lichtman, J. W., & Yuste, R. (2021). Ultrastructural analysis of dendritic spine necks reveals a continuum of spine morphologies. Developmental Neurobiology.
Pinel, P.J. & Barnes, S. (2021). Biopsychology (11th Edition). Boston. Pearson Education.
Sigurdsson, T., Doyère, V., Cain, C. K., & LeDoux, J. E. (2007). Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology, 52 (1), 215–227.
Squire, L. R. (1992). Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review, 99(2), 195–231.
Thompson, R. (1974). Localization of the “maze memory system” in the white rat. Physiological Psychology, 2 (1), 1-17.
Woodruff-Pak, D. S., Goldenberg, G., Downey-Lamb, M. M., Boyko, O. B., & Lemieux, S. K. (2000). Cerebellar volume in humans related to magnitude of classical conditioning. Neuroreport: For Rapid Communication of Neuroscience Research, 11(3), 609–615.
Yang, G., Pan, F., & Gan, W. B. (2009). Stably maintained dendritic spines are associated with lifelong memories. Nature, 462 (7275), 920-924.
Attributions
"Learning Objectives," "Overview," "The Search for Learning and Memory in the Synapse," "Changes at the Synapse Correlated with Learning and Memory," "Changes at the Synapse Correlated with Learning and Memory: Anatomical Changes in Dendritic Spines," "LTP, Neurochemical Cascades, and Stages of Memory," original material written by Kenneth A. Koenigshofer, Ph.D., is licensed under CC BY 4.0. Modified by Alan Keys, Ph.D., Sacramento City College, Sacramento, CA.
"Synaptic Plasticity," "Synaptic Plasticity: short-term enhancement, long-term potentiation and long-term depression," "Short-term Synaptic Enhancement and Depression," "Long-term Potentiation (LTP)," and "Long-term Depression (LTD)," adapted by Kenneth A. Koenigshofer, Ph.D., from Lumen Boundless Biology, How Neurons Communicate; https://courses.lumenlearning.com/bo...s-communicate/; curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike.
"Cellular and Receptor Level Mechanisms Revisited" adapted from The Brain from Top to Bottom; license: Copyleft, https://thebrain.mcgill.ca/flash/pop.../pop_copy.html; modified by Kenneth A. Koenigshofer, PhD., licensed under CC BY 4.0.

