8.2: Alcohol
- Page ID
- 171139
Alcohol (or more specifically, ethanol) is a drug that has a long history and is extensively used throughout the world. Before exploring the pharmacology of alcohol, we will start by learning more about its use, prevalence, legislation, chemical composition, and measurement.
11.1.1. History and Legislation
It is estimated that humans have been consuming alcohol for around 9000 years. Wine and beer existed in ancient Egyptian and Sumerian civilizations, with records dating as far back as 3200 B.C. Ancient Greeks used wine in religious ceremonies around 800 B.C., and Roman culture involved heavy drinking that persisted until the fall of Rome in 476 A.D. Distilled liquor first appeared around 1250 A.D., eventually followed by brandy, gin, and rum.
Alcohol is created by the fermentation of sugars from a variety of sources, such as grapes (wine), grains (beer), honey (mead), and sugarcane (rum). Methods of production spread throughout the world and contributed to its popularity. Alcoholic beverages were seen as more nutritious than water. Its consumption was also tied to social interaction, religious ceremonies, and celebrations, which increased its cultural importance.
Opposition to alcohol use has existed for as long as the recorded history of alcohol. In the United States, Dr. Benjamin Rush was a signer of the Declaration of Independence and was recognized by the American Psychiatric Association as the “Father of American Psychiatry”. He recognized alcoholism as a disease and was an early temperance leader. the movement to prohibit alcohol started to pick up steam in the 1800s. After the Civil War, traction for the movement increased, fueled largely by the Women’s Christian Temperance Union. The National Prohibition Act, also known as the Volstead Act, was passed in 1920 and added to the Constitution as the 18th Amendment. It banned the sale and distribution but not the consumption of alcohol across the U.S.
During the Prohibition era, consumption of alcohol was driven underground to speakeasies. At the same time, widespread smuggling of alcohol, known as bootlegging, arose to meet demand sparking an increase in organized crime. Support for prohibition steadily dropped, and 13 years later, it was repealed by the 21st Amendment in 1933. Although this marked the end of national prohibition, some counties and towns remained “dry” and continued to prohibit the sale of alcohol to this day. Individual states were assigned responsibility for regulating alcohol and setting their own legal drinking ages. The National Minimum Drinking Age Act, passed in 1984, ensured that sale was prohibited to anyone under the age of 21. Any state that did not comply with this legislation lost 10% of its federal highway funding. This was reinforced by the National Highway System Designation Act of 1995, which required that states adopt a “zero-tolerance law” to prohibit under-21 driving if the operator had at least a blood alcohol level of 0.02%.
11.1.2. Prevalence of Alcohol Use
Alcohol is one of the most commonly used drugs. According to the 2020 National Survey on Drug Use and Health (NSDUH), over half of Americans aged 12 or older reported that they drank in the past month. This widespread use of alcohol also contributes to risky behaviors. Binge drinking involves drinking large amounts of alcohol in a short time span (usually 4–5 drinks in about 2 hours) and carries a higher risk than normal drinking. Roughly 23% of people 18 and older reported binge drinking in the past month (SAMHSA, 2021).
Driving while intoxicated (DWI), also known as driving under the influence (DUI), is another common consequence of alcohol use. Vehicle collisions involving alcohol-impaired driving accounted for 29% of all driving fatalities in the U.S. in 2019. Similar to smoking, alcohol consumption also imparts considerable health risks to the user. Alcohol is the third-most preventable cause of death in the U.S, after smoking and poor diet (Mokdad et al., 2004).
Underage drinking is also common. According to the 2020 NSDUH, nearly 40% of people ages 12-20 reported drinking alcohol. Although young people drink less often than adults, they also engage in riskier behaviors. More than 90% of all alcoholic drinks consumed by young people involved binge drinking. Statistics show that at least 60% of undergraduate students acknowledge past-month consumption of alcohol.
11.1.3. Ethanol Properties and Concentrations
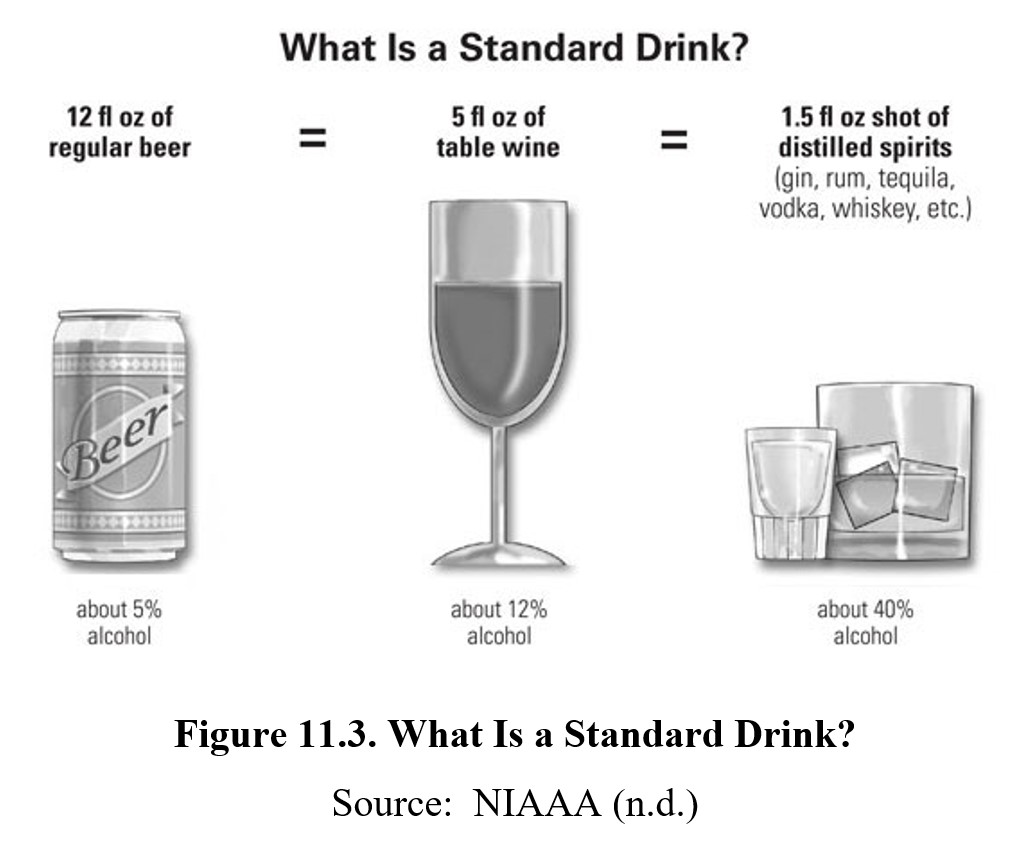
A common shorthand for determining alcohol consumption is a standard drink or an alcoholic drink equivalent. A standard drink contains about 0.6 fluid ouches or about 14 grams of pure ethanol. This amount of alcohol would be contained within a 12-oz can of beer (around 5% ethanol), a 5-oz glass of wine (around 12% ethanol), or a 1.5-oz shot of distilled spirits (upwards of 40% ethanol). Three standard drinks of any of these alcoholic beverages will usually put you over the legal limit for driving, though there is individual variability and even a single drink in some individuals can cause significant impairment.
11.2. ABSORPTION AND DISTRIBUTION
The pharmacokinetics of ethanol starts with its absorption and distribution. These topics are important to more than just neuroscience and health professional students. Understanding how alcohol is absorbed is necessary for responsible drinking. Laws that are written to punish drunk driving reference the concentration of ethanol in the blood.
11.2.1. Absorption of Ethanol
Ethanol is extremely soluble in both water and fat, making it readily absorbed through tissue. Ethanol is typically ingested orally through alcoholic drinks. These can also be administered rectally, although the practice is uncommon and not advised. Alcohol enemas results in faster absorption and higher blood concentrations because it bypasses the stomach. Another practice called vodka eyeballing involves introducing vodka into the eye sockets. Contrary to popular belief, eyeballing vodka does not induce rapid intoxication and runs the risk of corneal abrasions and scarring.
When taken orally, only 20-25% is absorbed by the stomach, while 75–80% is absorbed through the small intestine. The oral bioavailability of ethanol is poor for two reasons. First, there are alcohol-metabolizing enzymes found in the stomach wall. Second, any ethanol that is absorbed from the digestive tract enters the hepatic portal vein which goes straight to the liver. This will be explained further in the section on Metabolism of Ethanol.
The presence of food in the stomach delays transit from the stomach to the small intestine. This results in stomach enzymes having a longer time period to oxidize ethanol before it can enter the small intestine. Consequently, food reduces peak blood concentration. This is the reason why eating food while drinking is advised. Refer to the graph below to see the effect of food on ethanol absorption:
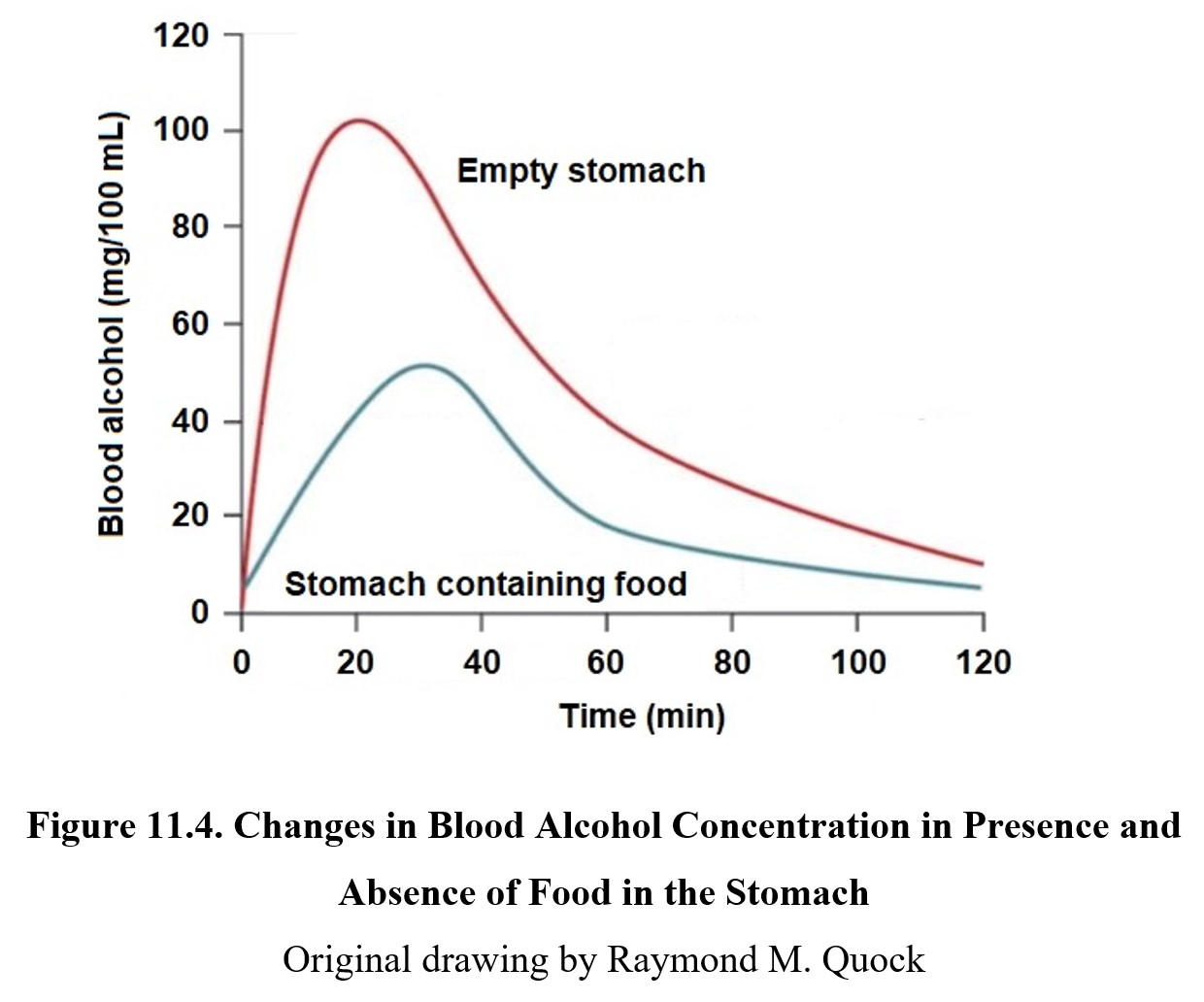
On an empty stomach, 50% of ethanol is absorbed in 15 minutes, with maximum blood levels being reached in 20 minutes. Most of the ethanol (80–90%) is absorbed within an hour or less of administration. The more food in the stomach, the less ethanol absorbed, although there is a gender difference. There is a gender difference in the activity of stomach and liver enzymes so that a larger amount of ethanol will be absorbed by women than men.
11.2.3. Blood Alcohol Concentration
The concentration of ethanol in the body is measured as blood alcohol concentration (BAC), also called blood alcohol content or blood alcohol level (BAL). BAC is defined as the number of grams of ethanol per 100 mL of blood. For example, a BAC of 0.05 is equivalent to 0.05 grams of ethanol per 100 mL of blood.
The legal limit for BAC while driving is 0.08, except in Utah where it is 0.05. For drivers under 21 years of age, most states have penalties for BAC above 0.02, although some states have stricter laws. BAC can be estimated by breathalyzer tests, which measure the amount of ethanol expelled through respiration. Although this is not identical to actual blood alcohol concentration, it is reasonably close in most circumstances and can be used as a basis for arrest.
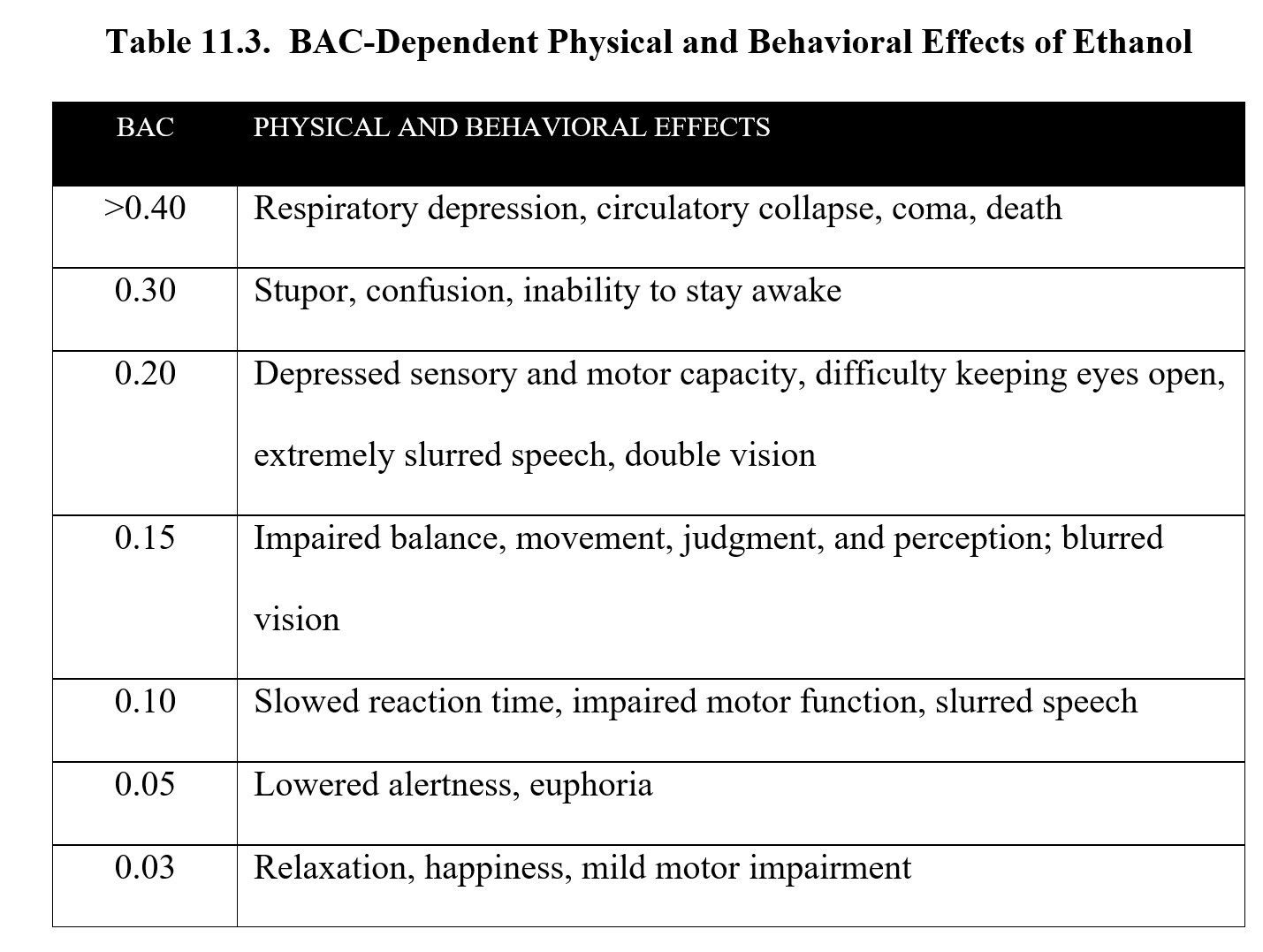
Below is a table of the effects of alcohol at different BACs. As you can see, although the legal limit is 0.08, impairment occurs at lower concentrations. Take a moment to study the contents of the table before moving on.
Blood Alcohol Concentration [5:27]
11.3. METABOLISM AND EXCRETION
11.3.1. Metabolism of Ethanol
Most of the ethanol ingested (around 90–95%) is metabolized by the body. The remainder is excreted as is in urine, feces, and other secretions. Some of the non-metabolized ethanol escapes via the lungs, which is why breathalyzers can measure BAC indirectly.
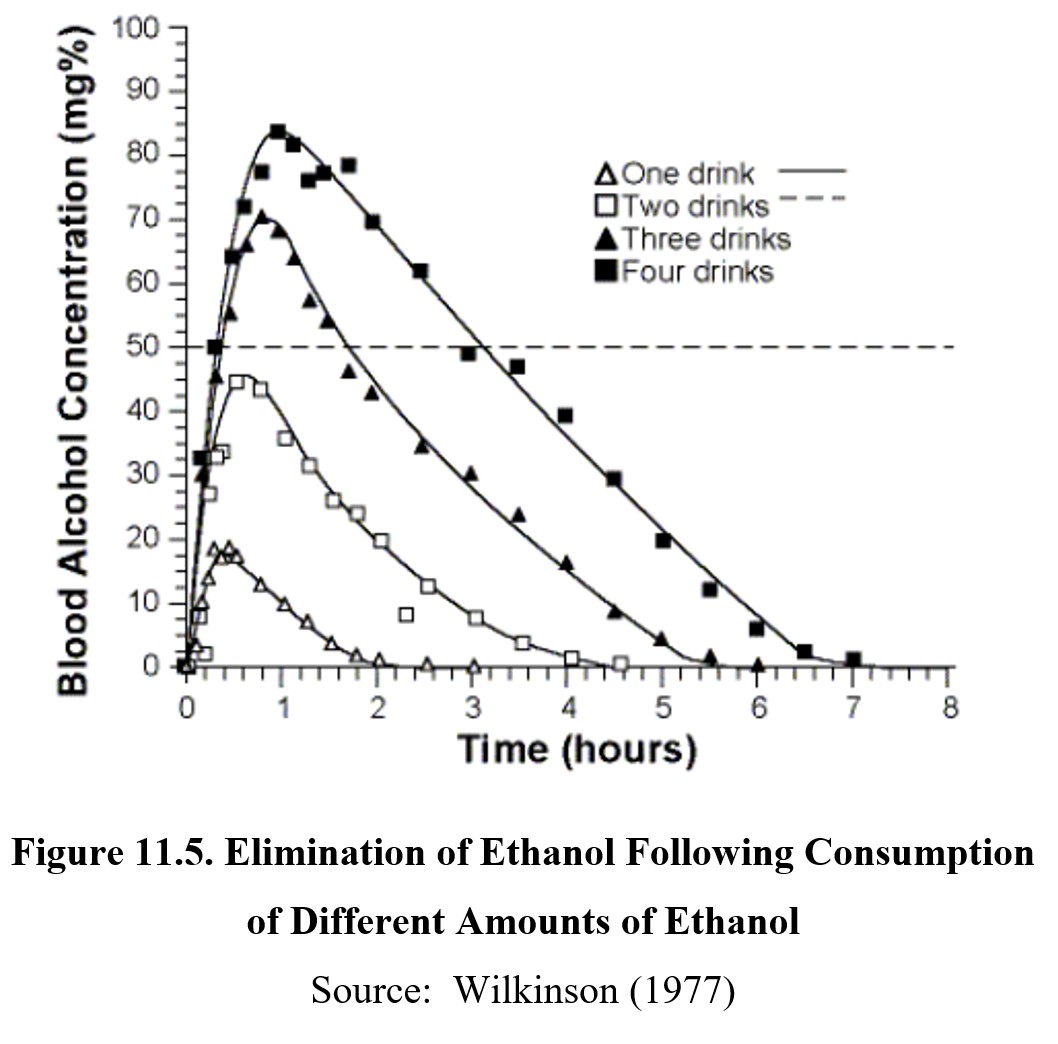
Ethanol is eliminated by zero-order kinetics. All the drugs we have covered so far followed first-order kinetics and have half-lives. Ethanol does not have a half-life because a fixed amount of it is metabolized per unit of time (note the relatively flat and parallel slopes of the graphs below). Therefore, drinking more alcohol does not speed up metabolism—with a few notable exceptions that we will get to later in this section. Typically, it takes about an hour for the body to clear itself of one standard drink of alcohol (i.e., 12-oz beer, 5-oz wine, or 1.5-oz distilled spirits).
11.4.1. Receptors and CNS Effects
Ethanol is considered a “dirty drug,” an informal term meaning that it affects many different receptors in the body and can produce a wide range of effects. The primary depressant mechanism of ethanol occurs at the GABAA chloride channel receptor complex. The exact site that ethanol binds to is unknown, as is its exact effect on chloride ion channels, but it enhances the effects of GABA and causes hyperpolarization.
The effects of ethanol are biphasic. At low doses, consumption of alcohol can elicit relaxation, giddiness, and euphoria. This is known as a buzz and is what makes initial drinking feel good. As BAC rises, depressant effects start to kick in. Motor coordination is impaired, speech becomes slurred, and sensory input is diminished. At high doses, ethanol can induce stupor, sleep, respiratory depression, coma, and death. (For a full list of effects at different BACs, check the table under the BAC subsection 11.2.3 above.)
11.4.2. Ethanol Dependence
Chronic ethanol use results in both tolerance and dependence. Tolerance develops in multiple ways. Standard pharmacodynamic tolerance occurs, which involves changes in target receptor regulation (neuroadaptation). Long-term exposure to ethanol causes the receptors to downregulate and become less responsive.
Pharmacokinetic tolerance also develops with heavy drinking. Recall that when ADH is saturated, the MEOS kicks in and CYP2E1 starts oxidizing excess ethanol. Unlike ADH, CYP2E1 and other cytochrome P450 enzymes can be induced, which results in faster metabolism of ethanol. This only occurs when ADH is saturated, so this type of tolerance only affects binge drinkers and other heavy users.
Finally, becoming familiar with the effects of inebriation leads to behavioral tolerance. This is a conditioned response to the drug that allows the user to compensate for its behavioral effects. People with behavioral tolerance can appear sober even after drinking a significant amount of alcohol and may have better control over their speech, movement, appearance, and emotional state compared to inexperienced drinkers.
Like other drugs, ethanol causes reward and reinforcement by altering dopamine transmission in the reward system. Normally, dopaminergic neurons in the VTA are under tonic inhibition by GABA interneurons. This means that ambient GABA activity somewhat inhibits these neurons from firing and releasing dopamine in the nucleus accumbens.
When ethanol is ingested, β-endorphin is released from an opioid nerve terminal. β-Endorphin, in turn, activates μ-opioid (mu-opioid) receptors on the GABA interneurons and inhibits the tonic release of GABA. This, in turn, depolarizes the dopaminergic neurons and increases dopamine release in the nucleus accumbens. This is the mechanisms that underlies the reward and reinforcing effects of ethanol. See the image below for the full process:
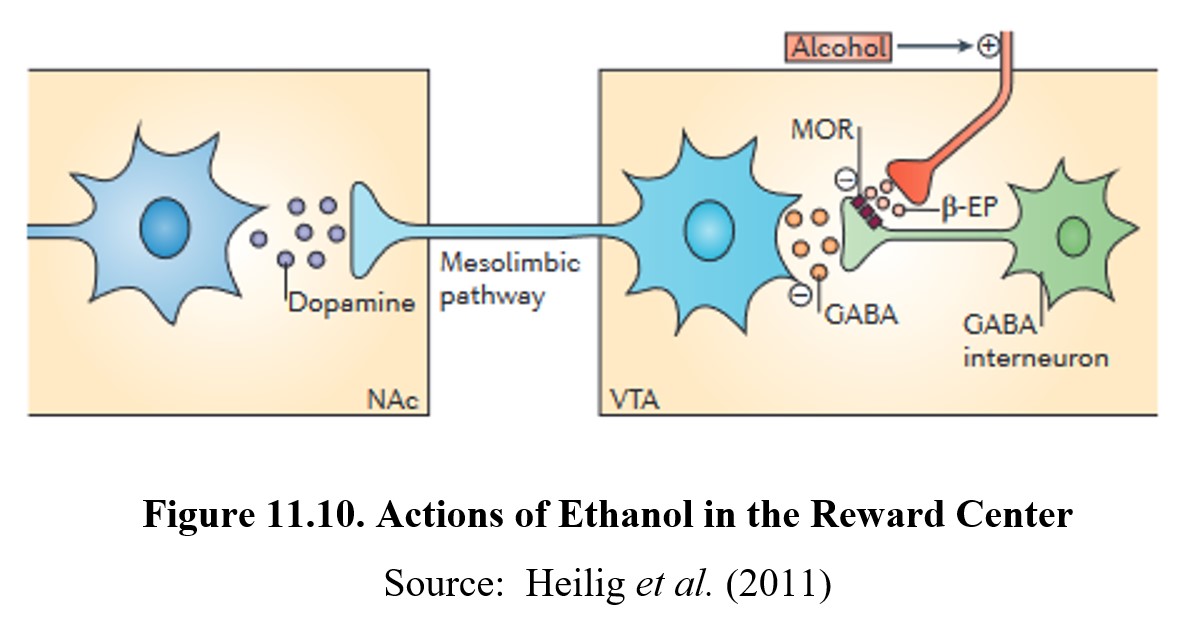
Ethanol withdrawal can be very dangerous and can last for days. Because chronic use decreases the number of GABAA receptors and increases NMDA receptors, CNS activity is significantly increased during withdrawal. Symptoms can include seizures, agitation, anxiety, increased heart rate and blood pressure, insomnia, nausea and vomiting, and paranoia. Again, we observe that signs of withdrawal proceed in the opposite direction from the effects of the drug. The intensity of the withdrawal is dependent on the duration and degree of physical dependence. During severe withdrawal, delirium tremens may occur, which involves shaking, confusion, and hallucinations and may lead to death. Delirium tremens usually occurs a few days after the onset of withdrawal and may last for two to three days.
Because ethanol withdrawal is so dangerous, treatment is usually intensive and requires hospitalization. Sedatives such as benzodiazepines are administered to mitigate withdrawal symptoms. Of those with severe symptoms, up to 15% may die from withdrawal.
11.4.3. Effects on Other Systems
Ethanol has considerable effects on systems in the body outside of the CNS. The first and foremost is the liver. The majority of ethanol is metabolized in the liver, which leads to a buildup of acetaldehyde in the liver. As mentioned before, acetaldehyde is toxic and can cause damage to the liver. Consuming large amounts of alcohol regularly will lead to a gradual deterioration of liver functioning known as alcoholic liver disease.
Ingestion of ethanol also affects the gastrointestinal system in a variety of ways. Low doses of ethanol accelerate gastric emptying, while moderate consumption stimulates digestive juices and acid secretion. At high doses, ethanol damages the lining of the stomach and causes inflammation, while also reducing the digestive system’s ability to absorb nutrients. This is the reason why physicians advise ulcer patients not to drink. Ethanol can impair the healing of the ulcer and may cause the ulcer to get worse.
The endocrine system, which is a series of glands that secrete hormones, is also altered by ethanol use. Ethanol increases blood sugar levels, which eventually results in the over-production of insulin and a subsequent decrease in blood sugar. If the patient is diabetic and receiving therapy with oral hypoglycemic drugs, ethanol is likely to complicate efforts to maintain normal blood glucose levels.
Ethanol interferes with the synthesis of Vitamin D, a hormone essential for absorption of calcium from the digestive tract. Without Vitamin D, the body’s calcium pool is diminished, affecting bone growth and remodeling. This can result in decreased bone density (osteoporosis) and an increased likelihood of broken bones.
Ethanol inhibits the release of vasopressin from the posterior pituitary gland. Vasopressin normally promotes water retention by the kidney and regulates the production of urine. In the absence of vasopressin, water is not reabsorbed back into the body and is eliminated as urine. This results in polyuria or the production of abnormally large volumes of dilute urine.
Ethanol suppresses testosterone production in males by interfering with gonadotropin release from the pituitary gland. Reduced testosterone also reduces the sperm count and causes erectile dysfunction.
The effect of ethanol on women’s health is also complicated. Ethanol can increase estrogen levels but can increase the risk of estrogen-dependent breast cancer in pre-menopause women. Ethanol also reduces progesterone levels which disrupts the normal menstrual cycle and causes low libido and hot flashes. In post-menopausal women, ethanol may reduce risks for coronary heart disease and osteoporosis.
Finally, excessive ethanol consumption may suppress the immune system. This manifests as a decrease in T cells, increased memory T cells, and higher rates of induced cell death. A weakened immune system causes increased susceptibility to bacterial infections such as pneumonia, tuberculosis, hepatitis, and septicemia.

Chapter 11: Alcohol by Washington State University is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted.

