3.4: Assigned Male at Birth Sexual Reproductive Anatomy
- Page ID
- 167177
Unique for its role in human reproduction, a gamete is a specialized sex cell carrying 23 chromosomes—one half the number in body cells. At fertilization, the chromosomes in one assigned male gamete, called a sperm (or spermatozoon), combine with the chromosomes in one assigned female gamete, called an oocyte. The function of the assigned male reproductive system, see Figure 27.2 is to produce sperm and transfer them to the assigned female reproductive tract. The paired testes are a crucial component in this process, as they produce both sperm and androgens, the hormones that support assigned male reproductive physiology. In humans, the most important assigned male androgen is testosterone. Several accessory organs and ducts aid the process of sperm maturation and transport the sperm and other seminal components to the penis, which delivers sperm to the assigned female reproductive tract. In this section, we examine each of these different structures, and discuss the process of sperm production and transport.
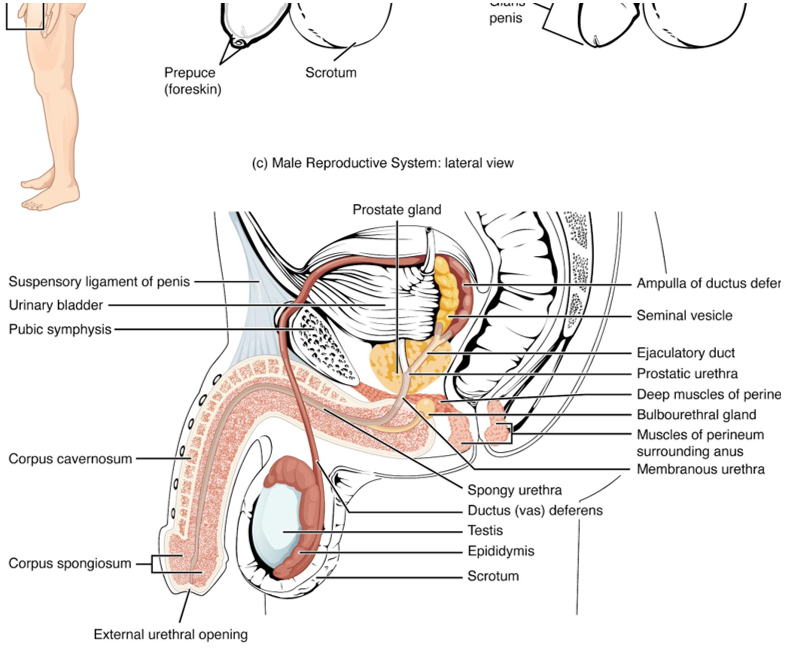
Scrotum
The testes are located in a skin-covered, highly pigmented, muscular sack called the scrotum that extends from the body behind the penis see Figure 27.2. This location is important in sperm production, which occurs within the testes, and proceeds more efficiently when the testes are kept 2 to 4°C below core body temperature.
The dartos muscle makes up the subcutaneous muscle layer of the scrotum, Figure 27.3. It continues internally to make up the scrotal septum, a wall that divides the scrotum into two compartments, each housing one testis. Descending from the internal oblique muscle of the abdominal wall are the two cremaster muscles, which cover each testis like a muscular net. By contracting simultaneously, the dartos and cremaster muscles can elevate the testes in cold weather (or water), moving the testes closer to the body and decreasing the surface area of the scrotum to retain heat. Alternatively, as the environmental temperature increases, the scrotum relaxes, moving the testes farther from the body core and increasing scrotal surface area, which promotes heat loss. Externally, the scrotum has a raised medial thickening on the surface called the raphae.
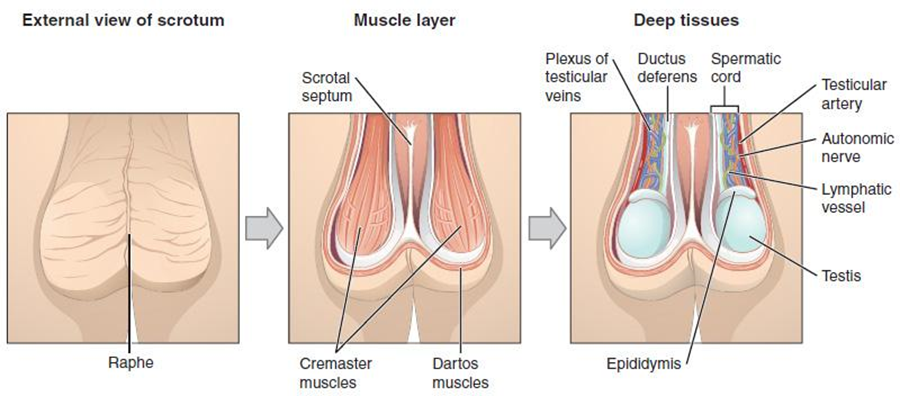
Testes
The testes (singular = testis) are the assigned male gonads—that is, the assigned male at birth’s reproductive organs. They produce both sperm and androgens, such as testosterone, and are active throughout the reproductive lifespan.
Paired ovals, the testes are each approximately 4 to 5 cm in length and are housed within the scrotum, see Figure 27.3. They are surrounded by two distinct layers of protective connective tissue (Figure 27.4). The outer tunica vaginalis is a serous membrane that has both a parietal and a thin visceral layer. Beneath the tunica vaginalis is the tunica albuginea, a tough, white, dense connective tissue layer covering the testis itself. Not only does the tunica albuginea cover the outside of the testis, it also invaginates (turns inside out or folds back on itself) to form septa that divide the testis into 300 to 400 structures called lobules. Within the lobules, sperm develop in structures called seminiferous tubules. During the seventh month of the developmental period of an assigned male fetus, each testis moves through the abdominal musculature to descend into the scrotal cavity. This is called the “descent of the testis.” Cryptorchidism is the clinical term used when one or both of the testes does not descend into the scrotum prior to birth.
The tightly coiled seminiferous tubules form the bulk of each testis. They are composed of developing sperm cells surrounding a lumen, the hollow center of the tubule, where formed sperm are released into the duct system of the testis. Specifically, from the lumens of the seminiferous tubules, sperm move into the straight tubules (or tubuli recti), and from there into a fine meshwork of tubules called the rete testes. Sperm leave the rete testes, and the testis itself, through the 15 to 20 efferent ductules that cross the tunica albuginea.
Inside the seminiferous tubules are six different cell types. These include supporting cells called sustentacular cells, as well as five types of developing sperm cells called germ cells. Germ cell development progresses from the basement membrane—at the perimeter of the tubule—toward the lumen. Let’s look more closely at these cell types.
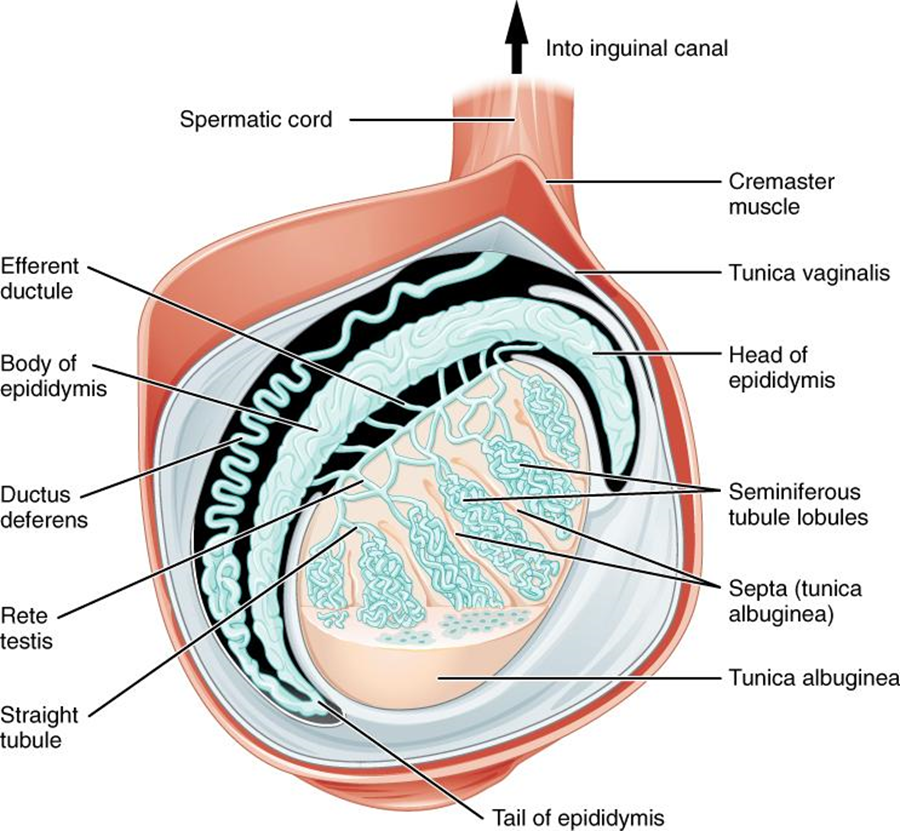
Sertoli Cells
Surrounding all stages of the developing sperm cells are elongate, branching Sertoli cells. Sertoli cells are a type of supporting cell called a sustentacular cell, or sustentocyte, that are typically found in epithelial tissue. Sertoli cells secrete signaling molecules that promote sperm production, and can control whether germ cells live or die. Tight junctions between these sustentacular cells create the blood–testis barrier, which keeps bloodborne substances from reaching the germ cells and, at the same time, keeps surface antigens on developing germ cells from escaping into the bloodstream, prompting an autoimmune response.
Germ Cells
The least mature cells, the spermatogonia (singular = spermatogonium), line the basement membrane inside the tubule. Spermatogonia are the stem cells of the testis, which means that they are still able to differentiate into a variety of different cell types throughout adulthood. Spermatogonia divide to produce primary and secondary spermatocytes, then spermatids, which finally produce formed sperm. The process that begins with spermatogonia and concludes with the production of sperm is called spermatogenesis.
Spermatogenesis
As just noted, spermatogenesis occurs in the seminiferous tubules that form the bulk of each testis (see Figure 27.4). The process begins at puberty, after which time sperm is produced constantly throughout life. One production cycle, from spermatogonia through formed sperm, takes approximately 64 days. A new cycle starts approximately every 16 days, although this timing is not synchronous across the seminiferous tubules. Sperm counts—the total number of sperm produced—slowly decline after age 35, and some studies suggest that smoking can lower sperm counts irrespective of age.
Two identical diploid cells result from spermatogonia mitosis. One of these cells remains a spermatogonium, and the other becomes a primary spermatocyte, the next stage in the process of spermatogenesis. As in mitosis, DNA is replicated in a primary spermatocyte, before it undergoes a cell division called meiosis I. During meiosis I each of the 23 pairs of chromosomes separates. This results in two cells, called secondary spermatocytes, each with only half the number of chromosomes. Now a second round of cell division (meiosis II) occurs in both of the secondary spermatocytes. During meiosis II each of the 23 replicated chromosomes divides, similar to what happens during mitosis. Thus, meiosis results in separating the chromosome pairs. Eventually, the sperm are released into the lumen, and are moved along a series of ducts in the testis toward a structure called the epididymis for the next step of sperm maturation.
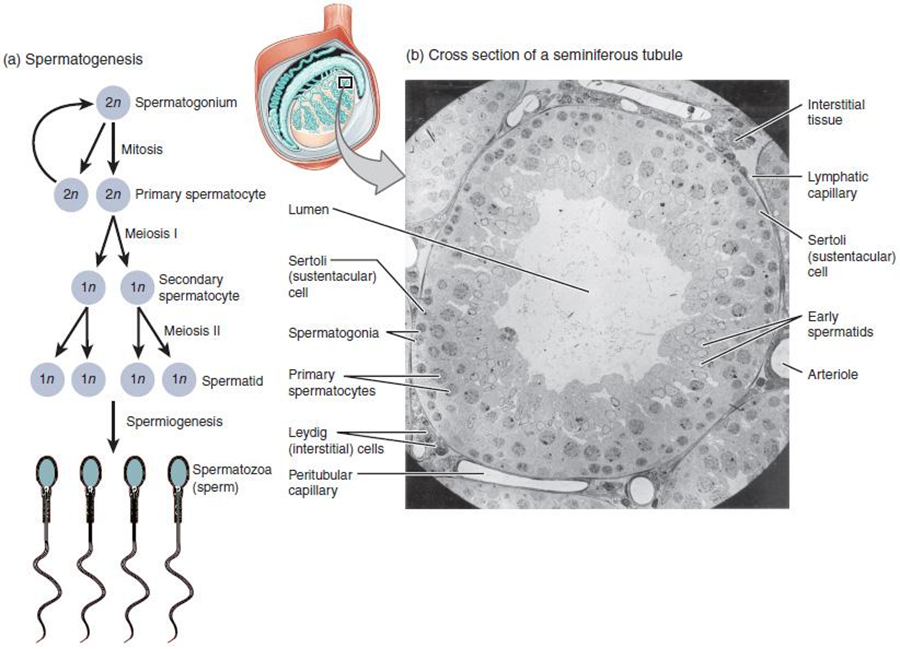
Structure of Formed Sperm
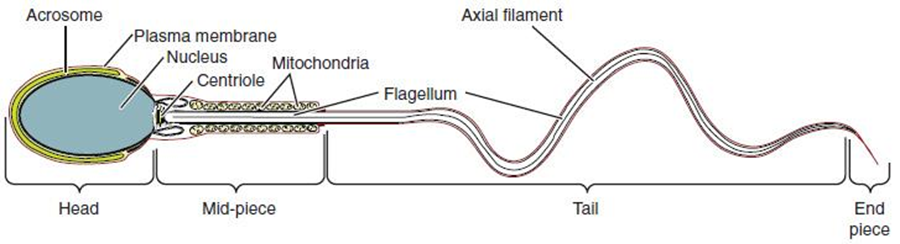
Sperm are smaller than most cells in the body; in fact, the volume of a sperm cell is 85,000 times less than that of the ovum. Approximately 100 million to 300 million sperm are produced each day, whereas the ovary typically releases only one oocyte per month. As is true for most cells in the body, the structure of sperm cells speaks to their function. Sperm have a distinctive head, mid-piece, and tail region (Figure 27.6). The head of the sperm contains the extremely compact haploid nucleus with very little cytoplasm. These qualities contribute to the overall small size of the sperm (the head is only 5 μm long). A structure called the acrosome covers most of the head of the sperm cell as a “cap” that is filled with lysosomal enzymes important for preparing sperm to participate in fertilization. Tightly packed mitochondria fill the mid-piece of the sperm. ATP produced by these mitochondria will power the flagellum, which extends from the neck and the mid-piece through the tail of the sperm, enabling it to move the entire sperm cell. The central strand of the flagellum, the axial filament, is formed from one centriole inside the maturing sperm cell during the final stages of spermatogenesis.
Sperm Transport
To fertilize an ovum, sperm must be moved from the seminiferous tubules in the testes, through the epididymis, and—later during ejaculation—along the length of the penis and out into the assigned female reproductive tract.
Role of the Epididymis
From the lumen of the seminiferous tubules, the immotile sperm are surrounded by testicular fluid and moved to the epididymis (plural = epididymides), a coiled tube attached to the testis where newly formed sperm continue to mature (see Figure 27.4). Though the epididymis does not take up much room in its tightly coiled state, it would be approximately 6 m (20 feet) long if straightened. It takes an average of 12 days for sperm to move through the coils of the epididymis, with the shortest recorded transit time in humans being one day. Sperm enter the head of the epididymis and are moved along predominantly by the contraction of smooth muscles lining the epididymal tubes. As they are moved along the length of the epididymis, the sperm further mature and acquire the ability to move under their own power. Once inside the assigned female reproductive tract, they will use this ability to move independently toward the unfertilized ovum. The more mature sperm are then stored in the tail of the epididymis (the final section), until ejaculation occurs.
Duct System
During ejaculation, sperm exit the tail of the epididymis and are pushed by smooth muscle contraction to the ductus deferens (also called the vas deferens). The ductus deferens is a thick, muscular tube that is bundled together inside the scrotum with connective tissue, blood vessels, and nerves into a structure called the spermatic cord (see Figure 27.2 and Figure 27.3). Because the ductus deferens is physically accessible within the scrotum, surgical sterilization to interrupt sperm delivery can be performed by cutting and sealing a small section of the ductus (vas) deferens. This procedure is called a vasectomy, and it is an effective form of birth control. Although it may be possible to reverse a vasectomy, clinicians consider the procedure permanent, and advise individuals to undergo it only if they are certain they no longer wish to have children.
Sperm make up only 5 percent of the final volume of semen, the thick, milky fluid that the penis ejaculates. The bulk of semen is produced by three critical accessory glands of the reproductive system: the seminal vesicles, the prostate, and the bulbourethral glands.
Seminal Vesicles
As sperm pass through the ampulla of the ductus deferens at ejaculation, they mix with fluid from the associated seminal vesicle (see Figure 27.2). The paired seminal vesicles are glands that contribute approximately 60 percent of the semen volume. Seminal vesicle fluid contains large amounts of fructose, which is used by the sperm mitochondria to generate ATP to allow movement through the assigned female reproductive tract.
The fluid, now containing both sperm and seminal vesicle secretions, next moves into the associated ejaculatory duct, a short structure formed from the ampulla of the ductus deferens and the duct of the seminal vesicle. The paired ejaculatory ducts transport the seminal fluid into the next structure, the prostate gland.
Prostate Gland
As shown in Figure 27.2, the centrally located prostate gland sits anterior to the rectum at the base of the bladder surrounding the prostatic urethra (the portion of the urethra that runs within the prostate). About the size of a walnut, the prostate is formed of both muscular and glandular tissues. It secretes an alkaline, milky fluid to the passing seminal fluid—now called semen—that is critical to first coagulate and then decoagulate the semen following ejaculation. The temporary thickening of semen helps retain it within the assigned female reproductive tract, providing time for sperm to utilize the fructose provided by seminal vesicle secretions. When the semen regains its fluid state, sperm can then pass farther into the assigned female reproductive tract.
The prostate normally doubles in size during puberty. At approximately age 25, it gradually begins to enlarge again. Abnormal growth of the prostate, or benign prostatic hyperplasia (BPH), can cause constriction of the urethra as it passes through the middle of the prostate gland, leading to a number of lower urinary tract symptoms, such as a frequent and intense urge to urinate, a weak stream, and a sensation that the bladder has not emptied completely.
Bulbourethral Glands
The final addition to semen is made by two bulbourethral glands (or Cowper’s glands) that release a thick, salty fluid that lubricates the end of the urethra and the vagina, and helps to clean urine residues from the penile urethra. The fluid from these accessory glands is released after the sexual arousal, and shortly before the release of the semen. It is therefore sometimes called pre-ejaculatory fluid . It is important to note that, in addition to the lubricating proteins, it is possible for bulbourethral fluid to pick up sperm already present in the urethra, and therefore it may be able to cause pregnancy.
Watch this video to explore the structures of the assigned male reproductive system and the path of sperm, which starts in the testes and ends as the sperm leave the penis through the urethra. Where are sperm deposited after they leave the ejaculatory duct?

The Penis
The penis is the organ of copulation (sexual intercourse). It is flaccid for non-sexual actions, such as urination, and turgid and rod-like with sexual arousal. When erect, the stiffness of the organ allows it to penetrate into the vagina and deposit semen into the assigned female reproductive tract.
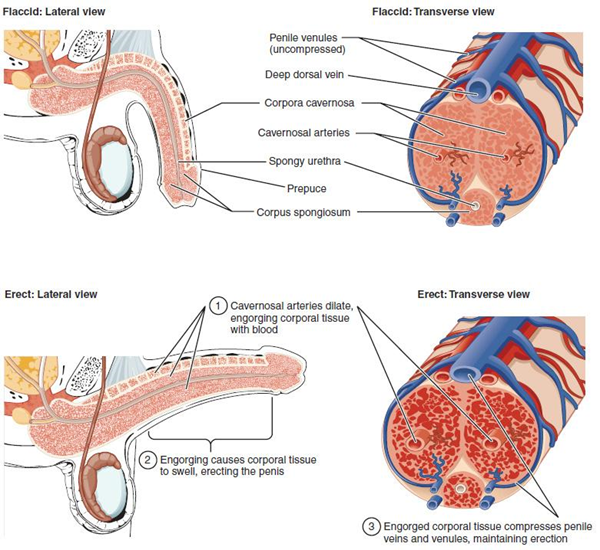
The shaft of the penis surrounds the urethra (Figure 27.7). The shaft is composed of three column-like chambers of erectile tissue that span the length of the shaft. Each of the two larger lateral chambers is called a corpus cavernosum (plural = corpora cavernosa). Together, these make up the bulk of the penis. The corpus spongiosum, which can be felt as a raised ridge on the erect penis, is a smaller chamber that surrounds the spongy, or penile, urethra. The end of the penis, called the glans penis, has a high concentration of nerve endings, resulting in very sensitive skin that influences the likelihood of ejaculation (see Figure 27.2). The skin from the shaft extends down over the glans and forms a collar called the prepuce (or foreskin). The foreskin also contains a dense concentration of nerve endings, and both lubricate and protect the sensitive skin of the glans penis. A surgical procedure called circumcision, often performed for religious or social reasons, removes the prepuce, typically within days of birth.
Both sexual arousal and REM sleep (during which dreaming occurs) can induce an erection. Penile erections are the result of vasocongestion, or engorgement of the tissues because of more arterial blood flowing into the penis than is leaving in the veins. During sexual arousal, nitric oxide (NO) is released from nerve endings near blood vessels within the corpora cavernosa and spongiosum. Release of NO activates a signaling pathway, which results in relaxation of the smooth muscles that surround the penile arteries, causing them to dilate. This dilation increases the amount of blood that can enter the penis and induces the endothelial cells in the penile arterial walls to also secrete NO and perpetuate the vasodilation. The rapid increase in blood volume fills the erectile chambers, and the increased pressure of the filled chambers compresses the thin-walled penile venules, preventing venous drainage of the penis. The result of this increased blood flow to the penis and reduced blood return from the penis is erection. Depending on the flaccid dimensions of a penis, it can increase in size slightly or greatly during erection, with the average length of an erect penis measuring approximately 15 cm or 5.9 inches.
"A circumcision operation, Nupe, North Nigeria" by Wellcome Collection is licensed by CC BY 4.0
Circumcision is a controversial procedure because it involves the mutilation of a penis. Many people view circumcision as a cosmetic procedure and argue that individuals should make their own decision regarding participating in the surgery once they reach their 18th birthday. Many cultures practice the ritual of circumcision, however, in recent decades; Australia, Canada, Ireland, New Zealand, and the United Kingdom have seen a decline in the ritual of circumcision.
Religion is typically a driving force in the decision to participate in circumcision, however, it is not always the case. While religion is a major driving force in the decision to circumcise a child, the decision to circumcise an individual can involve hygiene in addition to a way to express faith.
In recent decades, the potential risks associated with circumcision which include excessive bleeding, irritation to the head of the penis, the possibility of injury, or even a higher chance of meatitis (inflammation of the opening of the penis) have made many parents reconsider this elective surgical procedure.
Testosterone
Testosterone, an androgen, is a steroid hormone produced by Leydig cells. The alternate term for Leydig cells, interstitial cells, reflects their location between the seminiferous tubules in the testes. In assigned male embryos, testosterone is secreted by Leydig cells by the seventh week of development, with peak concentrations reached in the second trimester. This early release of testosterone results in the anatomical differentiation of the sexual organs. In childhood, testosterone concentrations are low. They increase during puberty, activating characteristic physical changes and initiating spermatogenesis.
Functions of Testosterone
The continued presence of testosterone is necessary to keep the reproductive system working properly, and Leydig cells produce approximately 6 to 7 mg of testosterone per day. Testicular steroidogenesis (the manufacture of androgens, including testosterone) results in testosterone concentrations that are 100 times higher in the testes than in the circulation. Maintaining these normal concentrations of testosterone promotes spermatogenesis, whereas low levels of testosterone can lead to infertility. In addition to intratesticular secretion, testosterone is also released into the systemic circulation and plays an important role in muscle development, bone growth, the development of secondary sex characteristics, and maintaining libido in both assigned males and assigned females. In assigned females, the ovaries secrete small amounts of testosterone, although most is converted to estradiol. A small amount of testosterone is also secreted by the adrenal glands in both assigned sexes.
Control of Testosterone
The regulation of testosterone concentrations throughout the body is critical for reproductive function. The intricate interplay between the endocrine system and the reproductive system is shown in Figure 27.8. The regulation of Leydig cell production of testosterone begins outside of the testes. The hypothalamus and the pituitary gland in the brain integrate external and internal signals to control testosterone synthesis and secretion. The regulation begins in the hypothalamus. Pulsatile release of a hormone called gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the endocrine release of hormones from the pituitary gland. Binding of GnRH to its receptors on the anterior pituitary gland stimulates release of the two gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These two hormones are critical for reproductive function in assigned males and assigned females. In assigned males, FSH binds predominantly to the Sertoli cells within the seminiferous tubules to promote spermatogenesis.
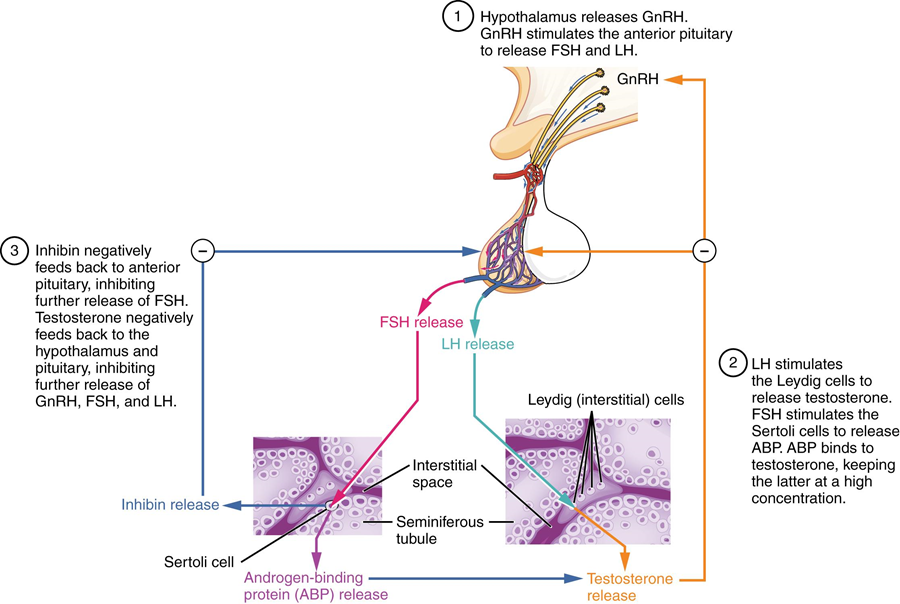
When concentrations of testosterone in the blood reach a critical threshold, testosterone itself will bind to androgen receptors on both the hypothalamus and the anterior pituitary, inhibiting the synthesis and secretion of GnRH and LH, respectively. When the blood concentrations of testosterone once again decline, testosterone no longer interacts with the receptors to the same degree, and GnRH and LH are once again secreted, stimulating more testosterone production. This same process occurs with FSH and inhibin to control spermatogenesis.
Review of Assigned Male Reproductive Anatomy
Gametes are the reproductive cells that combine to form a zygote. Organs called gonads produce the gametes, along with the hormones that regulate human reproduction. The male gametes are called sperm. Spermatogenesis, the production of sperm, occurs within the seminiferous tubules that make up most of the testis. The scrotum is the muscular sac that holds the testes outside of the body cavity.
Spermatogenesis begins with mitotic division of spermatogonia (stem cells) to produce primary spermatocytes that undergo the two divisions of meiosis to become secondary spermatocytes, then the haploid spermatids. During spermiogenesis, spermatids are transformed into spermatozoa (formed sperm). Upon release from the seminiferous tubules, sperm are moved to the epididymis where they continue to mature. During ejaculation, sperm exit the epididymis through the ductus deferens, a duct in the spermatic cord that leaves the scrotum. The ampulla of the ductus deferens meets the seminal vesicle, a gland that contributes fructose and proteins, at the ejaculatory duct. The fluid continues through the prostatic urethra, where secretions from the prostate are added to form semen. These secretions help the sperm to travel through the urethra and into the assigned female reproductive tract. Secretions from the bulbourethral glands protect sperm and cleanse and lubricate the penile (spongy) urethra.
The penis is the assigned male organ of copulation. Columns of erectile tissue called the corpora cavernosa and corpus spongiosum fill with blood when sexual arousal activates vasodilatation in the blood vessels of the penis. Testosterone regulates and maintains the sex organs and libido, and induces the physical changes of puberty. Interplay between the testes and the endocrine system precisely controls the production of testosterone with a negative feedback loop.


