3.3: Module 8 – Gender Through a Physiological Psychology Lens
- Page ID
- 150112
Module 8: Gender Through a Physiological Psychology Lens
Module Overview
We often hear of books such as “Men are from Mars and Women are from Venus” and “Men Are Like Waffles- Women Are Like Spaghetti: Understanding and Delighting in Your Difference” which suggest that men and women are opposites. While there is some disagreement as to how similar and different men and women truly are, the purpose of this module is to examine the biological differences in observed cognition, behavior, and gender roles with regards to one’s genes, hormones, and structure/function of the brain. We know that an individual’s genetic make-up, the production (or lack thereof) of hormones, as well as their brain anatomy can drastically impact their behavior. Therefore, the goal of this module is to explore the differences between men and women through a physiological psychology lens.
Module Outline
- 8.1. Basic Building Blocks
- 8.2. Endocrine System
- 8.3. Hormones
- 8.4. The Brain
Module Learning Outcomes
- To understand the relationship between DNA, genes, and chromosomes
- To gain a better understanding of the most common chromosomal abnormalities
- To better understand how the endocrine system functions and how the production of (or lack thereof) hormones impact ones social, cognitive, and behavioral development
- To understand gender differences in brain function and how this might impact differences in behavior
8.1. Basic Building Blocks
Section Learning Objectives
- To identify how genetic information is transferred from generation to generation
- To gain a better knowledge of both sex and non-sex linked chromosomal abnormalities
8.1.1. DNA
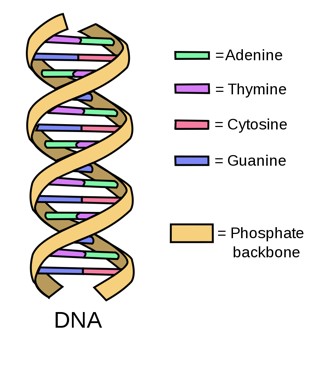
DNA, or deoxyribonucleic acid, is the most basic hereditary material in humans and most other organisms. Nearly every cell in your body (usually in the nucleus of the cell) contains some DNA which is comprised of four chemical bases: adenine (A), guanine (G), cytosine (C), and thymine (T). Each DNA base attaches to each other- A with T and C with G; these attached bases form base pairs. The backbone of DNA is comprised of a sugar and a phosphate molecule. One base pair along with its backbone form what is called a nucleotide. The nucleotides form two long strands that twist in a ladder-like structure forming a double helix (National Institute of Health, 2019).
8.1.2. Genes
While DNA neatly packages the hereditary material, genes are the basic physical and functional unit of heredity (National Institute of Health, 2019). DNA within each human gene varies from a few hundred DNA bases to more than 2 million bases! While most genes are the same among all individuals, there are a small number of genes (roughly 1%) that differ; these small differences are what make us all unique (National Institute of Health, 2019). Given the large number of DNA bases within a gene, you can imagine that “mistakes” or changes can occur. While some of these changes do not affect the individual, some can have catastrophic consequences. We will discuss this in more detail in section 8.1.3.1.
8.1.3 Chromosomes
We’ve already discussed that base pairs and a sugar/phosphate backbone create nucleotides. Several nucleotides form together to create DNA. Thousands to millions of DNA sequences create a gene. Hundreds to thousands of genes are packaged into chromosomes which are thread-like structures located inside the nucleus of a cell (National Institute of Health, 2019).
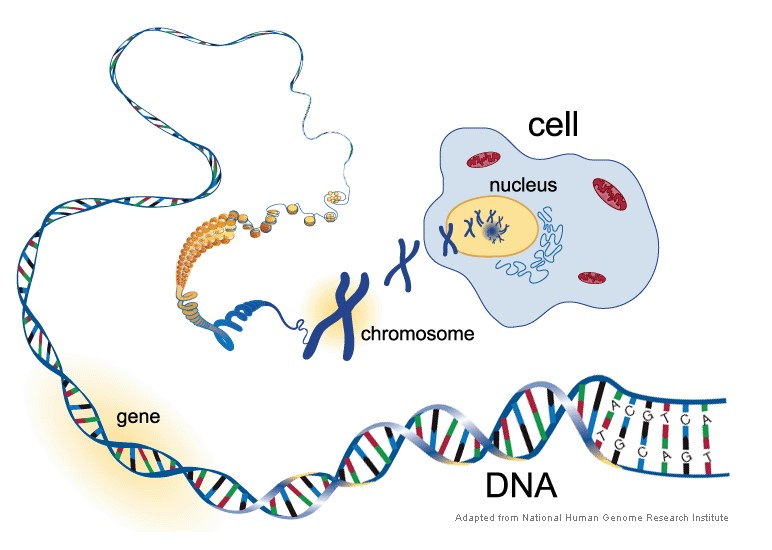
In humans, every cell should contain 23 pairs of chromosomes for a total of 46. Twenty-two of these pairs of chromosomes are autosomes, and one pair of chromosomes is an allosome. The 22 pairs of autosomes are (essentially) the same in males and females, however, the allosome, also known as the sex-chromosome, differs between males and females. Thus, this chromosome determines whether a fetus becomes genetically a male or female.
How does this happen? Males have one X chromosome and one Y chromosome, whereas females have TWO X chromosomes. The Y chromosome in males carries the genes that determine male sex. While other cells are produced by mitosis, gametes, or sex cells, are produced by meiosis cell division which results in the cells having only half of the number of chromosomes as the parent (National Institute of Health, 2019). Therefore, when the chromosomes split during meiosis, the two female cells each contain one X chromosome; however, when the male chromosomes split, one cell contains an X chromosome and the other contains a Y chromosome. Seeing as the Y chromosome contains the genetic information for the male sex, the sex of the fetus is determined by the male (father).
8.1.3.1. Chromosomal abnormalities. Chromosomal abnormalities occur when there is an anomaly, aberration, or mutation of chromosomal DNA (Genetic Alliance, 2009). This can occur during either egg/sperm development or during the early development of fetus. There are two ways in which these abnormalities can occur: numerical or structural. A numerical abnormality occurs when a whole chromosome is either missing OR an extra chromosome is attached to the pair. A structural abnormality occurs when part of an individual chromosome is missing, extra, or switched to another chromosome (Genetic Alliance, 2009). The range of effects of chromosomal abnormalities vary depending on the specific abnormality, with some as minimal as developmental delays to as severe as inability to sustain life.
We will now briefly discuss a few of the most common chromosomal abnormalities. While the first two chromosomal abnormalities are not sex-linked abnormalities, they are the most commonly observed, and therefore, worth mentioning. The final two chromosomal abnormalities are sex-linked and therefore, occur within specific genders.
Down syndrome (Trisomy 21). Down syndrome occurs when there is an extra chromosome on chromosome pair 21, hence the term trisomy 21. Trisomy 21 is the most common chromosomal condition in the United States and occurs in roughly 1 out of every 700 babies and generally effects males and females equally (Parker et al., 2010). Individuals with Down syndrome have distinct physical characteristics that include: flattened face, small head, short neck, protruding tongue, upward slanting eyelids, poor muscle tone, excessive flexibility, and shortened stature (National Library of Medicine, 2019). In addition, individuals with Down syndrome are more susceptible to congenital heart defects, gastrointestinal defects, sleep apnea, and dementia- with symptoms appearing around age 50. The lifespans of individuals with Down syndrome have increased dramatically over the years, with an average life expectancy of 60 years (National Library of Medicine, 2019).
The effects of the extra chromosome range from moderate to severe. Intellectual and developmental difficulties rage from mild to severe, however, research routinely supports the effectiveness of early intervention programs involvement in reduced developmental issues. Similarly, delayed developmental milestones are also common, usually related to low muscle tone. Early interventions with occupational, physical and speech therapists have been shown to reduce delay in both physical and speech development (National Library of Medicine, 2019).
While researchers are still unclear why this chromosomal abnormality occurs, advanced maternal age is one commonly identified risk factor for having a child with Down syndrome as the risk of conceiving a child with Down syndrome increases after 35 years of age. With that said, most children with Down syndrome are born to women under 35 years of age as there are more women having children prior to age 35 (National Library of Medicine, 2019).
Trisomy 18 (Edwards syndrome). Trisomy 18 occurs when there is a third chromosome on chromosome 18. A Trisomy 18 error occurs in about 1 out of every 2500 pregnancies in the US and 1 in 6000 live births (National Library of Medicine, 2019). The number of total births is higher because it includes a significant number of stillbirths that occur in the 2nd and 3rd trimesters of pregnancy.
Individuals with Trisomy 18 have significant medical complications that are potentially life-threatening, which is why this chromosomal abnormality has a high mortality rate. In fact, only 50% of babies that are carried to term will be born alive (National Library of Medicine, 2019). The birth rate is higher in baby girls than baby boys; baby girls also out-perform baby boys in the neonatal intensive care unit (NICU). Those born alive have a low birth rate due to slowed intrauterine growth. Physical abnormalities such as a small, abnormally shaped head, small jaw and mouth, clenched fists with overlapping fingers, as well as many other organ abnormalities are common in individuals with Trisomy 18 (Trisomy18 Foundation, 2019). Due to the severity of these abnormalities, only 5-10% of live births live to their first birthday. There have been rare cases of individuals with Trisomy 18 living into their twenties, however, they are unable to live independently without full time caregiving due to their significant developmental delays (Trisomy18 Foundation, 2019).
Klinefelter syndrome. Klinefelter syndrome is a rare sex chromosome disorder in males that occurs from the presence of an extra X chromosome. Individuals with Klinefelter syndrome have the normal XY chromosomes, plus an extra X chromosome for a total of 47 Chromosomes (XXY; National Library of Medicine, 2019). It is believed that the activity from the extra copy of multiple genes on the X chromosome disrupt many aspects of development from sexual development to developmental and physical development.
Occurring in about 1 in 650 newborn boys, Klinefelter syndrome is among the most common sex chromosome disorders. Symptoms can be so mild that the condition is not diagnosed until puberty or adulthood. In fact, researchers believe that up to 75% of affected individuals are never diagnosed (National Library of Medicine, 2019).
Individuals with Klinefelter syndrome typically have small testes that produce a reduced amount of testosterone. Because of the reduced hormone production, individuals with Klinefelter syndrome may have delayed or incomplete puberty, thus causing infertility. Unless treated with hormone replacement, the lack of testosterone can lead to breast enlargement, decreased muscle mass, decreased bone density, and a reduced amount of facial and body hair (National Library of Medicine, 2019).
Developmentally, individuals with Klinefelter syndrome often have learning disabilities, particularly with speech and language development. With that said, receptive language skills appear to supersede expressive language skills thus individuals with Klinefelter syndrome will understand speech but have difficulty communicating and expressing themselves (National Library of Medicine, 2019). Due to this language disruption, individuals with Klinefelter syndrome also often have difficulty learning to read.
While there are additional physical characteristics associated with Klinefelter syndrome, they are subtle. As adolescents and adults, these individuals may be taller than their peers. Children may have low muscle tone and problems with motor development such as sitting, standing, walking (National Library of Medicine, 2019). Similar to individuals with Down syndrome, early intervention programs are helpful in reducing the delay of motor development.
Psychiatrically, individuals with Klinefelter syndrome often experience anxiety, depression, and impaired social skills. There is a higher rate of ADHD and Autism Spectrum Disorder than that of the general public. Medically, they also experience complications related to metabolic issues (National Library of Medicine, 2019). More specifically half of men with Klinefelter syndrome develop conditions such as type 2 diabetes, hypertension (high blood pressure), and high cholesterol. They are also at an increased risk for developing osteoporosis, breast cancer, and autoimmune disorders compared to unaffected men (National Library of Medicine, 2019).
Turner syndrome. Unlike Klinefelter syndrome where there is an additional X chromosome, Turner syndrome occurs when there is one normal X chromosome and the other sex chromosome is missing or altered. Due to the altered X chromosome and lack of Y chromosome, individuals with Turner syndrome are genetically female. Turner syndrome is equally as rare as Klinefelter syndrome and occurs in about 1 in 2,500 newborn girls (National Library of Medicine, 2019).
Due to the altered or absence of the 2nd X chromosome, girls with Turner syndrome have a short stature which becomes apparent in early elementary years. Additional physical characteristics include low hairline at back of the neck, swelling of hands and feet, skeletal abnormalities, and kidney problems. Additionally, one third to half of girls born with Turner syndrome are born with a heart defect (National Library of Medicine, 2019).
Early developmental problems in girls with Turner syndrome vary significantly, with some experiencing developmental delays, nonverbal learning disabilities, and behavioral problems and others not requiring any early intervention. Despite these early developmental issues, girls and women with Turner syndrome typically have normal intelligence (National Library of Medicine, 2019).
Due to the altered sex chromosomes, women with Turner syndrome often experience early loss of ovarian function. While early prenatal development of ovaries is normal, egg cells die prematurely and majority of ovarian tissue degenerates before birth (National Library of Medicine, 2019). Due to ovarian loss, many affected girls do not undergo puberty unless they undergo hormone replacement therapy. Even with the hormone treatment, most women with Turner syndrome are unable to conceive children.
8.2. Endocrine System
Section Learning Objectives
- To identify the key organs involved in the endocrine system
- To understand the function of the endocrine system
- To understand why the endocrine system is important in behavior
8.2.1. Anatomy and Function
The endocrine system is made up of a network of glands that secrete hormones into the circulatory system that are then carried to specific organs (Tortora & Derrickson, 2012). While there are many glands that make up the endocrine system, it is often helpful to organize them by location. The hypothalamus, pituitary gland, and the pineal gland are in the brain; the thyroid and parathyroid glands are in the neck; the thymus is between the lungs; the adrenal glands are on top of the kidneys; and the pancreas is behind the stomach. Finally, the ovaries (for women) or testes (for men) are located in the pelvic region.
While all of the organs are important, there are two main organs that are responsible for the execution of the entire system: the hypothalamus and the pituitary gland. The hypothalamus is important because it connects the endocrine system to the nervous system. Its main job is to keep the body in homeostasis, or a balanced body state (Johnstone et al., 2014). When the body is out of balance, it is the hypothalamus’ job to identify the need (i.e. food to increase energy, water to increase hydration, etc.), and through the pituitary gland, identify how to achieve balance once again.
The pituitary gland is the endocrine system’s master gland. Through the help of the hypothalamus and the brain, the pituitary gland secretes hormones into the blood stream which “transmits information” to distant cells, regulating their activity (Johnstone et al., 2014). Non sex-related hormones that are released from the pituitary gland include: Growth hormone (GH), the hormone that stimulates growth in childhood and impacts healthy muscles and bones; Adrenocorticotropin (ACTH), the hormone responsible for production of cortisol which is activated in a stress response, and Thyroid-Stimulating hormone (TSH), the hormone responsible for regulating the body’s metabolism, energy balance, and growth. The pituitary gland also produces sex-related hormones that are involved in reproduction. For example, the pituitary gland is responsible for producing prolactin and oxytocin, which are both implicated in milk production for new mothers. Oxytocin may also play an important role in bonding between mother and child. Additional hormones including Luteinizing hormone (LH), which stimulates testosterone production in men and ovulation in women and Follicle-Stimulating hormone (FSH), which promotes sperm production in men and develops eggs in women are also maintained by the pituitary gland. LH and FSH work together to produce normal function of ovaries and testes. Deficits in any of these hormones may impact one’s reproductive ability.
Under the control of the hypothalamus and the pituitary gland, the remaining glands are responsible for manufacturing specific hormones that are carried throughout the body and help carry out specific functions. While it is beyond the scope of this course to identify all of the hormones and functions of the endocrine system, it is important to identify the five main functions of the endocrine system (Johnstone et al., 2014):
- Maintain homeostasis through the regulation of nutrient metabolism, water, and electrolyte balance.
- Regulate growth and production of cells.
- Control the responses of the body to external stimuli, especially stress.
- Control reproduction.
- Control and integrate circulatory and digestive activities with the autonomic nervous system.
8.2.2 Hypothalamus-pituitary-adrenal (HPA) Axis
As mentioned above, the endocrine system is involved in a lot of different body functions, however, one of the most important aspects of the endocrine system with regards to psychology is the HPA Axis. The HPA axis connects the central nervous system (brain and spinal cord) to the hormonal system. While there are many functions of this system, the most important (for this text purpose) is the stress-response system.
When in stress, the hypothalamus releases corticotropin-releasing hormone (CRH). CRH then activates the pituitary gland to release adrenocorticotropic hormone (ACTH). ACTH travels down to the adrenal gland on top of the kidneys which initiates the secretion of glucocorticoids from the adrenal cortex. The most common type of glucocorticoid in humans is cortisol, which plays a critical role in providing energy when presented with stressful or threatening situations (Kudielka & Kirschbaum, 2005). The elevated levels of cortisol produce a negative feedback loop, signaling brain functions to shut off the stress response system. A good video showing this response system can be found here https://www.neuroscientificallychallenged.com/glossary/hpa-axis.
As you will see more in other modules, particularly Module 10 when discussing clinical disorders, the HPA axis is responsible for keeping the body at homeostasis during stressful situations. A dysfunctional HPA axis has been associated with psychosomatic and mental health disorders. More specifically, HPA hyperactivity (i.e. too much activity) has been linked to major depression, whereas hypoactivity (i.e. too little activity) is associated with a host of autoimmune disorders, as well as fibromyalgia and chronic fatigue syndrome (Kudielka & Kirschbaum, 2005). Chronic HPA axis dysregulation has also been associated with the development of mood and anxiety disorders that will be discussed in more detail in Module 10.
8.2.2.1. Gender Differences in HPA Axis. Studies exploring the HPA axis hormonal response to stress among men and women have yielded conflicting findings. Kirschbaum and colleagues (1995a, b) identified higher cortisol and ACTH responses in men than women. Additional studies also found that men yielded greater cortisol and ACTH response to a psychological challenge (i.e. public speaking) than women. With that said, other studies have reported no significant gender differences in response to stress.
Some studies have indicated that a women’s menstrual cycle may be implicated in gender differences among activation of the HPA axis. For example, Kirschbaum and colleagues (1999) showed that free cortisol responses were similar between men and women in the luteal phase of their menstrual cycle, however, women in the follicular phase or those taking oral contraceptives showed less free cortisol compared to males. While there appears to be some biological differences in men and women’s activation of the HPA axis, one cannot rule out other factors such as cognitive appraisals that may also implicate individual differences in the stress response. These additional factors will be discussed in more detail in Module 10.
8.3. Hormones
Section Learning Objectives
- To understand the difference between estrogen and androgens
- To define and describe intersex conditions
- To identify the effect hormones have on social behavior and cognition
- To define and describe complete androgen insensitivity syndrome
The word hormone is derived from the Greek word meaning “arouse to activity.” Hormones are the body’s “chemical messengers.” Produced via the endocrine system, hormones travel throughout one’s bloodstream helping tissues and organs carry out their respected functions. Because there are so many types of hormones, they are often categorized by their function such as: reproduction/sexual differentiation; development and growth, maintenance of the internal environment, and regulation of metabolism/nutrient supply (Nussey & Whitehead, 2001). It should be noted that although hormones are categorized into these main groups, there are many hormones that affect more than one of these functions, and thus, serve multiple purposes.
8.3.1. Estrogens vs. Androgens
There are two classes of sex-related hormones: estrogens and androgens. Estrogens are hormones associated with female reproduction whereas androgens (i.e. testosterone) are associated with male reproduction. These hormones are not mutually exclusive, therefore, both males and females have both estrogen and androgens in their bloodstream—the difference is the amount of each hormone in each gender. For example, females have higher amounts of estrogen and lower amounts of androgens; males have higher amounts of androgens and lower amounts of estrogen.
Occasionally, there can be a disruption in hormone production causing an excess or reduction of hormone level. This disruption can lead to changes in the brain as well as physical changes. This can be especially problematic in sex-linked hormones as it can alter the production of both primary and secondary sexual characteristics depending on when the hormone imbalance occurs.
Primary sexual characteristics include sex organs that are needed for sexual reproduction. Secondary sexual characteristics are features that present during puberty and imply sexual maturation. Physical characteristics such as developing breasts, increased pubic hair, facial hair, widening of hips (women) and deepening of voice (males) are among of the most common secondary sexual characteristics. Due to hormonal disruption, occasionally there are situations where there is a discrepancy between one’s chromosomal sex and phenotypical sex (or external genitals). Known as intersex conditions, these situations have allowed researchers to study the effects of hormones on various behaviors.
8.3.2. Hormone Disorders
As previously discussed, occasionally there is a disruption in hormone production. This is seen in many medical disorders such as hyperthyroidism, dwarfism, and Cushing’s syndrome to name a few; however, sometimes there is a disruption in sex-related hormones. Below we will discuss the two most common types of conditions where sex-related hormones are affected and assess the implications of these disorders.
8.3.2.1. Congenital adrenal hyperplasia. One of the most common types of intersex conditions is Congenital Adrenal Hyperplasia (CAH). CAH is a genetic disorder that affects the adrenal glands and the production of hormones. More specifically, individuals with CAH have a significant enzyme deficiency that results in impaired synthesis of cortisol and aldosterone. The consistently low levels of cortisol results in an increase of ACTH by the pituitary gland which in response, causes an increase in synthesis of steroid precursors resulting in high androgen levels. While the hormonal effects of CAH can be of varying degrees, the most common, also known as Classic CAH, results in a complete lack of cortisol and an overproduction of androgens.
An individual with classic CAH will experience symptoms related to too little sodium in the body such as dehydration, poor feeding, low blood pressure, heart rate problems, and low blood sugar at birth (Mayoclinic, 2019). Due to the extensive nature of these symptoms, they are generally detected days or weeks within birth. In addition to the low cortisol related symptoms, individuals also experience effects related to high levels of androgens. Newborn females may present with ambiguous external genitalia despite having normal internal reproductive organs, whereas newborn males often have enlarged genitalia (Mayoclinic, 2019). Individuals with classic CAH will also experience significantly early onset of puberty—females may fail to menstruate or have irregular menstrual periods. Infertility in both males and females is also common.
Congenital Adrenal Hyperplasia has allowed researchers to study the effects of excess sex hormones on individual’s behaviors. While studied more extensively in females due to the fact that women do not usually develop high levels of androgens, findings suggest that prenatal exposure to excess androgen may influence the development of regions in the brain responsible for sex difference behaviors (Dittman et al., 1990). For example, some studies have found higher levels of energy and aggressive behaviors, an increase participation in sports, and an increased interest in traditionally masculine games and behaviors in girls with CAH (Berenbaum & Hines, 1992; Berenbaum & Snyder, 1995; Berenbaum, 1999).
These findings have been replicated over the years with CAH females routinely displaying more male-typical play behaviors in childhood. Assessment of CAH females as they age into adulthood also suggest differences in sexual identity. More specifically, CAH females report less satisfaction with their female sex assignment as well as less heterosexual interest than unaffected women. When assessing the relationship between childhood play and adult sexual preference in CAH females, a significant relationship was observed between increased male-typical play in childhood and decreased satisfaction with the female gender. These findings were also found between increased male-typical play in childhood and reduced heterosexual interest in adulthood (Hines, Brook, & Conway, 2004). Studies assessing behavior and sexual orientation in males with CAH have failed to identify any significant differences between males with CAH and unaffected males. These results are not surprising given the fact that unaffected males have higher levels of androgens than unaffected females.
8.3.2.2.Complete androgen insensitivity syndrome. Unlike CAH where there is an overproduction of androgens, Complete Androgen Insensitivity Syndrome (CAIS) is a rare condition that inhibits boys from responding to androgens. Occurring in approximately 2-5 per 100,000 births, individuals with CAIS are genetically male (XY), however, due to the body’s inability to respond to androgens, they display mostly female external sex characteristics. Despite the external female sex characteristics, these individuals are still genetically male and therefore, lack a uterus but do have undescended testes. While genetic testing in fetuses has expanded over the years, many individuals with CAIS are not diagnosed until menses fail to develop at puberty. While gender identity issues are likely, individuals with this syndrome are often raised female due to the external sexual characteristics at birth.
Physically, individuals with CAIS are generally taller than women without the disorder, but shorter than males. It is believed that part of this increased height is due to the effect of the growth controlling region on the long arm of the Y chromosome. There is little research on the psychological gender development of individuals with CAIS, however, the limited information available suggests that individuals with CAIS usually assume a gender identity and sexual orientation in line with their female sex rearing (Wisniewski et al., 2000). Individuals with CAIS report maternal interests and report high femininity from childhood to adulthood on global rating scales (Wisniewski et al., 2000). Psychologically, individuals with CAIS report similar levels of psychological well-being and overall quality of life as unaffected women. Similarly, there were no differences in psychological and behavioral domains suggesting CAIS women and unaffected women experience similar levels of psychological and behavioral symptoms (Hines, Ahmed, & Hughes, 2003).
8.3.3. Effects of Hormones on Behavior
We just briefly discussed how an atypical hormone levels via hormone disorders can have an effect on behavior, but what about the effect of typically producing hormones on men and women’s behaviors? Let’s take a look at how estrogen and testosterone can impact the way we behave!
8.3.3.1 Estrogen. Changing levels of estrogen across the reproductive lifespan have been associated with changes in incidence of anxiety in women. More specifically, women are more at risk for developing an anxiety disorder during onset of puberty, which is also associated with an increase of circulating estradiol from prepubertal to adult levels (Ojeda & Bilger, 2000). Furthermore, an increase in anxiety symptoms is also observed when estradiol levels drop post-menopause (Sahingoz, Ugus, & Gezginc, 2011). In women with anxiety disorders, there is an increase in anxiety symptoms during the luteal phase of the menstrual cycle, which is characterized by a dramatic decline in circulating estradiol levels (Cameron, Kuttesch, McPhee, & Curtis, 1988). Therefore, there appears to be a strong link between anxiety related behaviors and estradiol levels in women.
With the strong link between estrogen and anxiety related disorders, some argue that use of estrogen in treatment of these symptoms would be beneficial. Unfortunately, research exploring the use of various levels of estrogen to manage anxiety symptoms in rats has yielded conflicting evidence, thus has not been explored in humans (Kastenberger, Lutsch, & Schwarzer, 2012).
8.3.3.2. Testosterone. While estrogen has been linked to increased anxiety type behaviors, testosterone has most commonly been linked to aggression. Popular opinion suggests that testosterone is responsible for aggressive, violent, and other machismo behaviors, however, research suggests there is actually little empirical support for these assumptions (Booth, et al., 2006). In fact, this relationship is best explained by a bi-directional relationship that is dependent on many different intrinsic individual factors (Sapolsky, 1997). More specifically, it is assumed that testosterone increases the likelihood that certain (aggressive) behaviors will be expressed, if the individual’s intrinsic factors as well as the social contextual demands support the expression of this behavior (Booth et al., 2006). Therefore, testosterone alone is not responsible for aggressive behaviors, however, it may contribute to aggressive acts if other personal characteristics and environmental factors simultaneously occur.
A large meta-analysis indicated a weak positive relationship between testosterone and aggression suggesting individuals with higher levels of testosterone engage in more aggressive behaviors (Book, Starzyk & Quinsey, 2001). Interestingly, the largest effect in their findings was in males age 13-20 years of age. One possible explanation of increased aggressive behaviors during this age is a combination of increased testosterone due to puberty, in combination with increased impulsivity due to lack of prefrontal cortex development (Terburg, Morgan & van Honk, 2009). Furthermore, findings indicated that the most aggressive behaviors were related to a combination of high testosterone and low cortisol (Terburg, Morgan & van Honk, 2009). Low cortisol levels have long been implicated in aggressive and externalizing behavioral disorders.
Not surprisingly, aggressive behaviors have been examined in male prisoners. Findings routinely support the relationship between increased testosterone and more aggressive offenses (Dabbs et al., 1995). More specifically, testosterone levels are higher among men who committed personal crimes of sex and violence than those who committed property crimes of burglary, theft, or drugs (Dabbs et al., 1995). Furthermore, individuals with higher levels of testosterone were also more likely to have violated prison rules during their time served than their peers with lower levels of testosterone.
While there appears to be a relationship between testosterone and aggressive behavior, it is difficult to imply a causal role in aggression. Therefore, we must identify other possible explanations. Competitiveness of a situation is one variable researchers have explored. In examining aggressive behaviors during competitive video gaming, researchers found men made higher unprovoked attacks during the game than women. Furthermore, individuals with higher levels of testosterone also completed higher unprovoked attacks than those with lower levels of testosterone. From these findings, researchers imply that situational factors such as a threat to status or competition must interact with hormones to produce aggressive behaviors (McAndrew, 2009).
Another possible explanation for the sex-difference in aggressive behaviors is emotional arousal. More specifically, males are more easily aroused than females and more importantly, are often less able to regulate their emotions (Knight et al., 2002). This theory is supported by research identifying no sex differences in aggressive behaviors when arousal was zero, a large sex difference in small to medium arousal levels, and again no difference when there was high arousal (Campbell, 2006). Researchers assumed the sex-difference in aggressive behaviors at small and medium arousal levels was due in large part to impulsivity, with men being more impulsive than women.
8.3.4. Effects of Hormones on Cognition
Sex hormones influence cognition at many stages of life, however, the focus of most of the research is the relationship between estrogen and testosterone and the decline of cognition in older age. General findings suggest that estrogen may serve as a protective factor in cognitive decline in elderly women, whereas lack of testosterone in men may be linked to a general decline in cognition. In this section we will discuss the implications of hormones on men and women’s cognitive functioning throughout the lifespan.
Studies in women have identified a relationship between specific brain regions and estrogen. More specifically, the prefrontal cortex and the hippocampus have been identified as areas that improve in function due to increased estrogen (Hara, Waters, McEwen & Morrison, 2015). The hippocampus, the brain region responsible for memory and learning, appears to be affected by stress differently in men and women. Researchers found that women have a heightened sensitivity to stress within the hippocampus region. For example, ten days of a significant stressor in men causes the opioid system within the hippocampus to “shut down,” whereas in women, the system is “primed.” This priming encourages excitement and learning when the individual is exposed to activation of the opioid system again (Marrocco & McEwen, 2016). As you will see in Module 10, this may have implication on the development of psychological symptoms post stressful situations (i.e. depression, anxiety, PTSD) as women’s systems may have learned to fear a specific situation due to the “primed” system.
Endocrine changes appear to be largely responsible for age-related cognitive decline in both men and women (Henderson, 2008). In women, the most significant change in hormones occurs during menopause. While menopause can occur naturally, it can also be medically induced via the removal of the ovaries and uterus due to a variety of reasons (i.e. cancer, pregnancy complications, etc.). Research examining cognitive effects in women experiencing either natural or medically induced menopause indicates that regardless of the menopause method, women are at an increased risk for cognitive decline once menopause is “complete.” Interestingly, cognitive decline in women who undergo menopause due to medical necessity respond to estrogen replacement, whereas those who undergo menopause naturally do not respond as favorably to estrogen support (Phillips & Sherwin, 1992). It should be noted that although cognitive declines due to reduced estrogen are observed, they are often mild and are generally observed as deficits in concentration and processing speed (Kok et al., 2006).
When examining the relationship between men and cognitive decline, testosterone has been identified as a variable that may significantly impact performance on a variety of cognitive tasks. For example, men with low levels of testosterone have been shown to perform lower on cognitive tasks such as memory (Barrett-Connor et al., 1999), executive functioning (Muller et al., 2005), and attention (Cappa et al., 1998). The effects of testosterone on these cognitive tasks appear to have a greater effect when assessed in elderly men; results on the effects of testosterone and cognition does not appear to impact young men (Yaffe et al., 2002; Barret-Connor et al., 1999).
Similar to that in women, researchers have also examined outcomes in performance with supplementation of testosterone in older men experiencing low levels of testosterone. Findings indicate that supplementation of testosterone is an effective method to improve working memory and other cognitive function in older men (Janowsky, Chavez, & Orwoll, 2000; Cherrier et al., 2001). It should be noted, however, that despite the support for increased testosterone and cognitive function, researchers are still unsure of how much testosterone is needed for “optimal” cognitive performance (Barrett-Connor et al., 1999).
8.4. The Brain
Section Learning Objectives
- To identify gender differences in the lateralization of the brain
- To identify gender differences in cortical thickness of the brain
- To identify gender differences in myelination of the brain
Another attempt to explain sex differences is through the anatomy of the brain. Sexual dimorphism, or the “condition where two sexes of the same species exhibit different characteristics beyond the differences in their sexual organs” (https://en.Wikipedia.org/wiki/Sexual_dimorphism), has long suggested that differences in brain anatomy, size, and volume among genders are responsible for the differences in behavior. The purpose of this section is to explore these brain differences and determine how they may impact men and women’s behavior.
8.4.1 Lateralization
The brain is divided into two hemispheres and connected via the corpus collosum. The right hemisphere is thought to be dominant in spatial abilities whereas the left hemisphere is dominant in verbal tasks. While early researchers proposed that women were more right brained and men were more left brain, their theory has been continually refuted over the years with many studies suggesting that both genders utilize both brain hemispheres equally (Bishop & Wahlsten, 1997).
So, if men and women use both sides of their brain equally, maybe men and women utilize the brain regions differently? More specifically, what if women were more bilateral (utilizing both hemispheres) whereas men were more lateralized, thus using each hemisphere for distinct functions? Again, researchers failed to support these findings consistently. One consistent argument against the bilateral argument is if women were more bilateral, one would expect women to have a larger corpus collosum due to the increased communication between the hemispheres. Unfortunately, while some studies have found an increased corpus collosum size in women, others have found no significant difference between genders (Steinmetz et al., 1995).
Given these findings, are there any differences between genders regarding lateralization of brain? Despite the lack of evidence for sex differences in lateralization, there is some support for lateralization of spatial skills. Findings indicate that men would use their right hemisphere whereas women were more bilateral (Vogel, Bowers, & Vogel, 2003). Thus, many researchers fail to find a difference in hemisphere use between the genders, however, of those that do, they are consistent in that men appear to utilize their right hemisphere, whereas women are more bilateral.
8.4.2 Cortical Thickness
Cortical thickness, or the tissue volume and tissue composition of the cerebrum, has long been explored as a possible explanation for behavioral differences in men and women. Magnetic Resource Image (MRI) studies have shown that gray matter, white matter, and brain size are smaller in women than men, even after controlling for body size. When both gray and white matter normalize, adult men have a greater proportion of white matter, whereas women demonstrate a greater proportion of gray matter (Allen et al., 2003; Gur et al., 1999). Women also demonstrate significantly greater global and regional cortical thickness, while no significant thickening is observed in men. This significant cortical thickening in women is localized in anatomical regions consistent with studies that support sexual dimorphism (Kiho et al., 2006).
During childhood and adolescence, white matter volume increases faster in boys than in girls. When examining specific brain regions, greater diffusivity was found in the corticospinal tract and the frontal white matter in the right hemisphere for boys, whereas greater diffusivity was found in the occipital-parietal regions and the most superior aspect of the corticospinal tracts in the right hemisphere in girls (Rabinowicz, Dean, Petetot, & de Courten-Myers, 1999). Coincidently, girls show a greater organization in the right hemisphere compared to the left hemisphere for boys. These differences in brain matter and diffusivity may indicate differing developmental trajectories for both boys and girls, as well as possibly explain gender-specific abilities and/or behavioral differences between sexes.
8.4.3 Myelination
Myelination, or the development of an insulating myelin sheath around nerves so they can transmit information more quickly, develops earlier in boys than girls. More specifically, by the age of two, myelination of long fiber tracks in the brain is more developed in males than females, thus allowing information to transmit faster in males.
One particular study examined brain density changes in girls and boys through childhood and adolescents. The findings from the study indicated that boys showed significantly greater loss of grey matter volume and an increase in both white matter and corpus collosum area compared with girls over a similar age range. Girls did show significant developmental changes with age, but at a slower rate than boys (DeBellis et al., 2001). The researchers argue that grey matter decreases are likely to reflect dendritic pruning which typically occurs during puberty. Dendritic pruning essentially eliminates extra neurons and synaptic connections to increase the efficiency of neuronal transmissions. It is suspected that the white matter density increase is related to increased myelination and/or axonal size, which also helps improve the efficiency of neuronal transmission.
Another aspect of myelination that appears to be different in men and women is related to Multiple Sclerosis (MS). MS is a chronic inflammatory disease of the central nervous system that causes inflammation, demyelination and axonal damage, leading to a wide range of neurological symptoms. It is found to be more prevalent in women. In fact, women are two to three times more likely to be diagnosed with MS than men. While the ultimate cause of MS is damage to the myelin, nerve fibers, and neurons in the brain and spinal cord, the onset of this degeneration is unknown. There is suspect that it is a combination of both genetic and environmental factors, however, further research is needed on this disease (National Multiple Sclerosis Society, 2019).
Regardless of these anatomical differences between males and females, it is important to note that difference in brain structure does not translate into a sex difference in brain function (DeVries & Sodersten, 2009). In fact, researchers observe activation of different brain areas in men and women when they are performing the same task. Therefore, differential activation does not always translate to differential performance. Further support for this finding comes from studies that observe men and women utilizing different strategies to complete the same task. Due to differences in strategies, it is not surprising that different brain regions are activated in men and women on the same task. Finally, it is important to remember that the brain is not constant and that behavior also has an impact on brain activation.
Module Recap
Module 8 explored the biological differences between males and females. We are all comprised of billions of cells that contain DNA, genes, and chromosomes. While most of our genetic make-up is the same, there are some small differences that lead to significant physical differences. We learned that occasionally, cell division can go array and chromosomal abnormalities can occur. We briefly discussed some of the most common sex and non-sex linked chromosomal abnormalities.
We discussed the importance of the endocrine system and how the HPA axis responds to stressful situations. We identified different anatomy that is involved in regulating hormones- both for sexual reproduction and basic bodily function (homeostasis). Hormones can have significant implications on behavior and we discussed the literature on the relationship between sex hormones and men and women’s behaviors and cognition. Finally, we discussed differences in brain structure and function in men and women. Although sex differences in brain anatomy and function are not clear, there are some implications for differences in male and female brains that may account for behavior differences between genders.


