18.14: Chapter 14- Mirror Neurons, Theory of Mind, Social Cognition, and Neuroscience of Its Disorders
- Page ID
- 113231
This page is a draft and under active development. Please forward any questions, comments, and/or feedback to the ASCCC OERI (oeri@asccc.org).
- Describe mirror and canonical neurons, their locations in the brain, and their possible functions
- Describe theory of mind (ToM) and its role in social cognition
- Define the social brain
Overview
In the early 1990s, researchers working with monkeys identified a group of neurons in their frontal cortex that were activated when the monkeys made a particular motion with their hand or mouth. There was nothing surprising in that, since these neurons were located in what is considered a “motor” portion of the brain. What was surprising, though, was that this same group of neurons was also activated in monkeys who were not performing the motion themselves, but rather watching other monkeys perform it. Hence these neurons were given the name “mirror neurons”. Mirror neurons are one possible basis in the brain for what may be called social intelligence or social cognition, information processing necessary for successful interactions with conspecifics. The new field of social neuroscience studies the neurological structures and processes that carry out the information processing required for adaptive interactions with others, basis for all human social behavior. One of these capacities, labeled “theory of mind,” is ability to perceive and understand mental states of others, essential in human social life. Autism is in the category of pervasive developmental disorders and may result in part from impaired theory of mind and abnormalities in a number of brain structures collectively referred to by some neuroscientists as the "social brain."
Are Mirror Neurons the Basis for Communication?
Mirror neurons were discovered in area F5 of the ventral premotor cortex of macaque monkeys by researchers at the University of Parma, Italy, in 1992. The researchers found that these neurons had some very distinctive characteristics : they fired not only when a monkey performed a voluntary gesture (for example, turned a handle to open a door), but also when a monkey watched another monkey perform this same action. The part of the brain where these mirror neurons are found in monkeys corresponds to the part of the human brain known as Broca’s area, which since the 19th century has been known to play an important role in language. In addition to their being located in a brain area associated with language in humans, two other things about mirror neurons have led many researchers to suggest that they may play a role in the evolution and learning of language: these neurons tell us about the intentions of the people around us, and they help us to imitate the movements of other people’s lips and tongues.
Another category of neurons present in area F5, canonical neurons, may, like mirror neurons, be involved in human language faculties. The special characteristic of canonical neurons is that they fire when an individual simply sees a graspable object. For example, if a monkey looks at a ball, the canonical neurons that fire are the same ones that will fire if the monkey decides to actually grasp the ball. In contrast, the monkey’s mirror neurons will not be activated by the mere sight of a ball, but only if the monkey either grasps the ball or sees another monkey do so.
In essence, mirror neurons react to visual stimuli that represent an interaction between a biological means of action (such as the hand or mouth) and an object. These neurons thus act as agents for recognizing purposive actions as opposed to simple movements.
Even in the case of canonical neurons, which can be activated by the sight of a graspable object in the absence of movement, the internal representation is that of a purposive action, and not just a simple movement of the hand or arm.
This is what has led some researchers to think that mirror neurons might help to explain the cognitive foundations of language, by providing the neural substrate for the human ability to understand the meaning of other people’s actions, which is the basis for all social relations. This system of correspondences between perceptions and actions would help us to infer other people’s mental states and to interpret their actions as intentional behaviors arising from these states. We can then easily imagine how this mechanism for interpreting gestural communication might have been applied to verbal communication as well.
The hypothesis advanced is that the motor system, through its mirror neurons, is involved in perceiving speech, and that through evolution, the “motor resonance” generated by the mirror neurons has been diverted (or exapted ) from its original function to serve the needs of language. One has to be impressed by the economy of such a cognitive system, in which one individual understands what other individuals are doing (or saying) on the basis of the internal neural representation of his or her own motor capabilities.
The point is that intentional communication between two individuals differs from the simple cries of alarm by which animals signal danger to all members of their group indiscriminately. Intentional communication, in contrast, requires one individual who is transmitting information and a second who is paying attention to receive it. Among all the possible origins of language , the first form of intentional communication among humans may have arisen from the imitation of gestures and facial expressions. Thus mirror neurons may have played a role in sharing these common representations and, eventually, a common language.
Mirror neurons have been shown to have some other interesting characteristics as well. First of all, as noted above, they will be activated when you see someone else’s hand grasp an object, but not when you see a tool grasp the same object. The explanation is that unlike tools and other human artifacts, human body parts are represented in the motor and premotor areas of the brain’s frontal lobes. Second—and here is where the implications for language become really interesting—mirror neurons do not react to just any movements of the hand or mouth, but only to movements that are involved in goal-directed actions.
| In other words, it is only when an action has a meaning that it activates the mirror neurons. Their response is thus associated with the expression of intentionality—that is, with the purpose of the observed gesture. For example, certain mirror neurons that are activated when a monkey manipulates an object will remain quiet when the monkey uses the same muscles, connected to the same neurons, to perform a similar action for a different purpose, such as to scratch itself or to pick an insect out of its fur. |
It is becoming more and more apparent that the brain’s motor system does not only control movements but can also in a certain sense read the actions performed by other individuals. Mirror neurons may thus play a fundamental role in all human social behavior, including language.
Summary
Mirror neurons discovered in area F5 of the frontal cortex in the macaque monkey, an area that corresponds to Broca's area of the left frontal lobe of humans, fire action potentials when a monkey performs a voluntary movement (for example, turning a handle to open a door), but also when a monkey watches another monkey perform this same action. if a monkey looks at a ball, the canonical neurons that fire are the same ones that will fire if the monkey decides to actually grasp the ball. However, by contrast, the monkey’s mirror neurons will not be activated by the mere sight of a ball, but only if the monkey either grasps the ball or sees another monkey do so. Mirror neurons do not react to just any movements of the hand or mouth, but only to movements that are involved in goal-directed actions. These neurons thus act as agents for recognizing purposive actions as opposed to simple movements. The hypothesis is that the motor system, through its mirror neurons, is involved in perceiving speech, and the intentions of others, and may thus play a fundamental role in all human social behavior.
Attributions
Are Mirror Neurons the Basis for Communication? by Bruno Dubuc, The Brain from Top to Bottom under a Copyleft license.
The Social Brain and Social Neuroscience
The brain mechanisms of social cognition and social intelligence help us understand other people and accomplish adaptive interactions with them. Deficits in these abilities due to damage in the "social brain" can lead to disorders such as autism.
Social Neuroscience
By Tiffany A. Ito and Jennifer T. KubotaUniversity of Colorado Boulder, University of Delaware
This module provides an overview of the new field of social neuroscience, which combines the use of neuroscience methods and theories to understand how other people influence our thoughts, feelings, and behavior. The module reviews research measuring neural and hormonal responses to understand how we make judgments about other people and react to stress. Through these examples, it illustrates how social neuroscience addresses three different questions: (1) how our understanding of social behavior can be expanded when we consider neural and physiological responses, (2) what the actual biological systems are that implement social behavior (e.g., what specific brain areas are associated with specific social tasks), and (3) how biological systems are impacted by social processes.
Learning Objectives
- Define social neuroscience and describe its three major goals.
- Describe how measures of brain activity such as EEG and fMRI are used to make inferences about social processes.
- Discuss how social categorization occurs.
- Describe how simulation may be used to make inferences about others.
- Discuss the ways in which other people can cause stress and also protect us against stress.
Psychology has a long tradition of trying to better understand how we think and act. For example, in 1939 Heinrich Kluver and Paul Bucy removed (i.e. lesioned) the temporal lobes in some rhesus monkeys and observed the effect on behavior. Included in these lesions was a subcortical area of the brain called the amygdala. After surgery, the monkeys experienced profound behavioral changes, including loss of fear. These results provided initial evidence that the amygdala plays a role in emotional responses, a finding that has since been confirmed by subsequent studies (Phelps & LeDoux, 2005; Whalen & Phelps, 2009).
What Is Social Neuroscience?
Social neuroscience seeks to understand how we think about and act toward other people. More specifically, we can think of social neuroscience as an interdisciplinary field that uses a range of neuroscience measures to understand social behavior. As such, social neuroscience studies the same topics as social psychology, but does so from a multilevel perspective that includes the study of the brain and body. Figure 1 shows the scope of social neuroscience with respect to the older fields of social psychology and neuroscience. Although the field is relatively new – the term first appeared in 1992 (Cacioppo & Berntson, 1992) – it has grown rapidly, thanks to technological advances in brain science, and to the recognition that neural and physiological information are critical to understanding how we interact with other people.
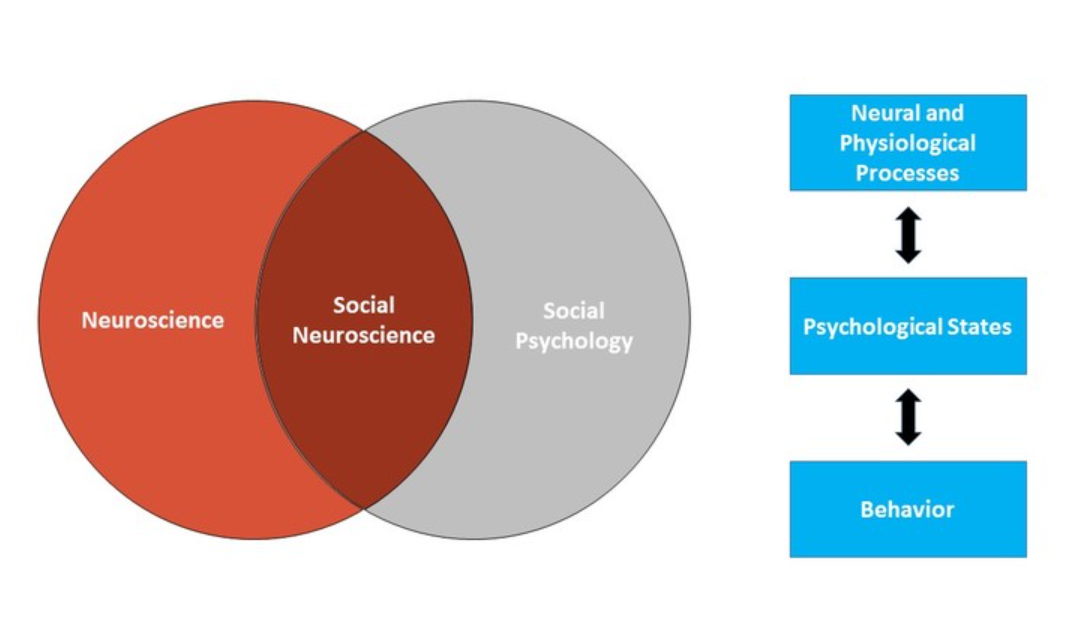 Figure \(\PageIndex{1}\): Social neuroscience is the intersection of social psychology and neuroscience. Under this multilevel approach, neural/physiological processes and behavior are two things we can measure or observe. Psychological states cannot be directly observed, but understanding them is the goal. Social neuroscientists use the observable neural/physiological processes and behavioral responses to make inferences about unobservable psychological states. The bidirectional arrows show that all levels of analysis are assumed to influence each other (e.g., psychological states can influence neural responses, and neural responses can influence psychological states).
Figure \(\PageIndex{1}\): Social neuroscience is the intersection of social psychology and neuroscience. Under this multilevel approach, neural/physiological processes and behavior are two things we can measure or observe. Psychological states cannot be directly observed, but understanding them is the goal. Social neuroscientists use the observable neural/physiological processes and behavioral responses to make inferences about unobservable psychological states. The bidirectional arrows show that all levels of analysis are assumed to influence each other (e.g., psychological states can influence neural responses, and neural responses can influence psychological states).Social neuroscience can be thought of as both a methodological approach (using measures of the brain and body to study social processes) and a theoretical orientation (seeing the benefits of integrating neuroscience into the study of social psychology). The overall approach in social neuroscience is to understand the psychological processes that underlie our social behavior. Because those psychological processes are intrapsychic phenomena that cannot be directly observed, social neuroscientists rely on a combination of measurable or observable neural and physiological responses as well as actual overt behavior to make inferences about psychological states (see Figure 1). Using this approach, social neuroscientists have been able to pursue three different types of questions: (1) What more can we learn about social behavior when we consider neural and physiological responses? (2) What are the actual biological systems that implement social behavior (e.g., what specific brain areas are associated with specific social tasks)? and (3) How are biological systems impacted by social processes?
How Automatically Do We Judge Other People?
Social categorization is the act of mentally classifying someone as belonging in a group. Why do we do this? It is an effective mental shortcut. Rather than effortfully thinking about every detail of every person we encounter, social categorization allows us to rely on information we already know about the person’s group. For example, by classifying your restaurant server as a man, you can quickly activate all the information you have stored about men and use it to guide your behavior. But this shortcut comes with potentially high costs. The stored group beliefs might not be very accurate, and even when they do accurately describe some group members, they are unlikely to be true for every member you encounter. In addition, many beliefs we associate with groups – called stereotypes – are negative. This means that relying on social categorization can often lead people to make negative assumptions about others.
The potential costs of social categorization make it important to understand how social categorization occurs. Is it rare or does it occur often? Is it something we can easily stop, or is it hard to override? One difficulty answering these questions is that people are not always consciously aware of what they are doing. In this case, we might not always realize when we are categorizing someone. Another concern is that even when people are aware of their behavior, they can be reluctant to accurately report it to an experimenter. In the case of social categorization, subjects might worry they will look bad if they accurately report classifying someone into a group associated with negative stereotypes. For instance, many racial groups are associated with some negative stereotypes, and subjects may worry that admitting to classifying someone into one of those groups means they believe and use those negative stereotypes.

Social neuroscience has been useful for studying how social categorization occurs without having to rely on self-report measures, instead measuring brain activity differences that occur when people encounter members of different social groups. Much of this work has been recorded using the electroencephalogram, or EEG. EEG is a measure of electrical activity generated by the brain’s neurons. Comparing this electrical activity at a given point in time against what a person is thinking and doing at that same time allows us to make inferences about brain activity associated with specific psychological states. One particularly nice feature of EEG is that it provides very precise timing information about when brain activity occurs. EEG is measured non-invasively with small electrodes that rest on the surface of the scalp. This is often done with a stretchy elastic cap, like the one shown in Figure 2, into which the small electrodes are sewn. Researchers simply pull the cap onto the subject’s head to get the electrodes into place; wearing it is similar to wearing a swim cap. The subject can then be asked to think about different topics or engage in different tasks as brain activity is measured.
To study social categorization, subjects have been shown pictures of people who belong to different social groups. Brain activity recorded from many individual trials (e.g., looking at lots of different Black individuals) is then averaged together to get an overall idea of how the brain responds when viewing individuals who belong to a particular social group. These studies suggest that social categorization is an automatic process – something that happens with little conscious awareness or control – especially for dimensions like gender, race, and age (Ito & Urland, 2003; Mouchetant-Rostaing & Giard, 2003). The studies specifically show that brain activity differs when subjects view members of different social groups (e.g., men versus women, Blacks versus Whites), suggesting that the group differences are being encoded and processed by the perceiver. One interesting finding is that these brain changes occur both when subjects are purposely asked to categorize the people into social groups (e.g., to judge whether the person is Black or White), and also when they are asked to do something that draws attention away from group classifications (e.g., making a personality judgment about the person) (Ito & Urland, 2005). This tells us that we do not have to intend to make group classifications in order for them to happen. It is also very interesting to consider how quickly the changes in brain responses occur. Brain activity is altered by viewing members of different groups within 200 milliseconds of seeing a person’s face. That is just two-tenths of a second. Such a fast response lends further support to the idea that social categorization occurs automatically and may not depend on conscious intention.
Overall, this research suggests that we engage in social categorization very frequently. In fact, it appears to happen automatically (i.e., without us consciously intending for it to happen) in most situations for dimensions like gender, age, and race. Since classifying someone into a group is the first step to activating a group stereotype, this research provides important information about how easily stereotypes can be activated. And because it is hard for people to accurately report on things that happen so quickly, this issue has been difficult to study using more traditional self-report measures. Using EEGs has, therefore, been helpful in providing interesting new insights into social behavior.
Do We Use Our Own Behavior to Help Us Understand Others?
Classifying someone into a social group then activating the associated stereotype is one way to make inferences about others. However, it is not the only method. Another strategy is to imagine what our own thoughts, feelings, and behaviors would be in a similar situation. Then we can use our simulated reaction as a best guess about how someone else will respond (Goldman, 2005). After all, we are experts in our own feelings, thoughts, and tendencies. It might be hard to know what other people are feeling and thinking, but we can always ask ourselves how we would feel and act if we were in their shoes.
There has been some debate about whether simulation is used to get into the minds of others (Carruthers & Smith, 1996; Gallese & Goldman, 1998). Social neuroscience research has addressed this question by looking at the brain areas used when people think about themselves and others. If the same brain areas are active for the two types of judgments, it lends support to the idea that the self may be used to make inferences about others via simulation.
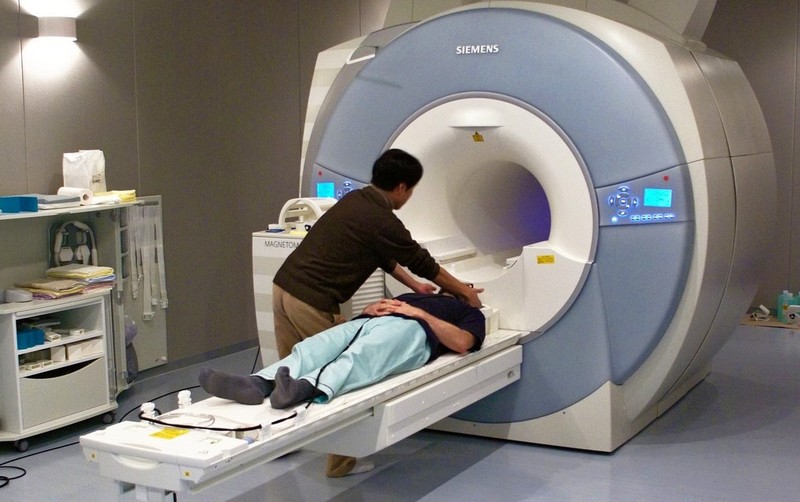
We know that an area in the prefrontal cortex called the medial prefrontal cortex (mPFC) – located in the middle of the frontal lobe – is active when people think about themselves (Kelley, Macrae, Wyland, Caglar, Inati, & Heatherton, 2002). This conclusion comes from studies using functional magnetic resonance imaging, or fMRI. While EEG measures the brain’s electrical activity, fMRI measures changes in the oxygenation of blood flowing in the brain. When neurons become more active, blood flow to the area increases to bring more oxygen and glucose to the active cells. fMRI allows us to image these changes in oxygenation by placing people in an fMRI machine or scanner (Figure 3), which consists of large magnets that create strong magnetic fields. The magnets affect the alignment of the oxygen molecules within the blood (i.e., how they are tilted). As the oxygen molecules move in and out of alignment with the magnetic fields, their nuclei produce energy that can be detected with special sensors placed close to the head. Recording fMRI involves having the subject lay on a small bed that is then rolled into the scanner. While fMRI does require subjects to lie still within the small scanner and the large magnets involved are noisy, the scanning itself is safe and painless. Like EEG, the subject can then be asked to think about different topics or engage in different tasks as brain activity is measured. If we know what a person is thinking or doing when fMRI detects a blood flow increase to a particular brain area, we can infer that part of the brain is involved with the thought or action. fMRI is particularly useful for identifying which particular brain areas are active at a given point in time.
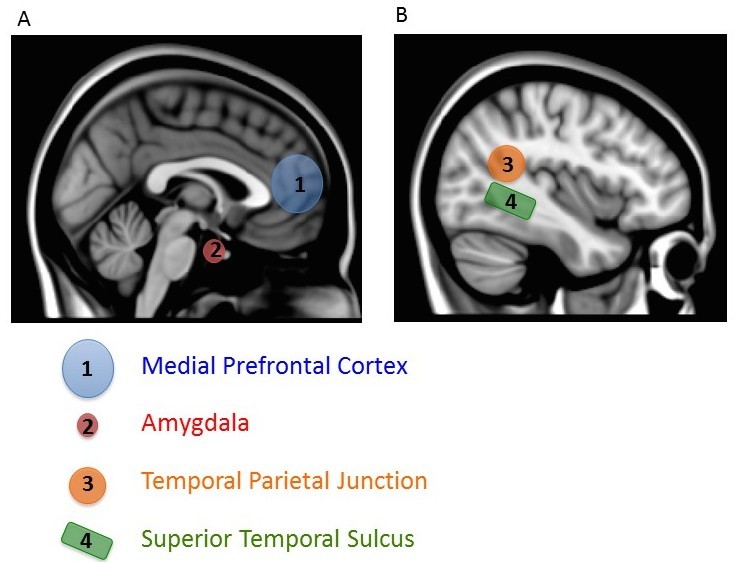
The conclusion that the mPFC is associated with the self comes from studies measuring fMRI while subjects think about themselves (e.g., saying whether traits are descriptive of themselves). Using this knowledge, other researchers have looked at whether the same brain area is active when people make inferences about others. Mitchell, Neil Macrae, and Banaji (2005) showed subjects pictures of strangers and had them judge either how pleased the person was to have his or her picture taken or how symmetrical the face appeared. Judging whether someone is pleased about being photographed requires making an inference about someone’s internal feelings – we call this mentalizing. By contrast, facial symmetry judgments are based solely on physical appearances and do not involve mentalizing. A comparison of brain activity during the two types of judgments shows more activity in the mPFC when making the mental versus physical judgments, suggesting this brain area is involved when inferring the internal beliefs of others.
There are two other notable aspects of this study. First, mentalizing about others also increased activity in a variety of regions important for many aspects of social processing, including a region important in representing biological motion (superior temporal sulcus or STS), an area critical for emotional processing (amygdala), and a region also involved in thinking about the beliefs of others (temporal parietal junction, TPJ) (Gobbini & Haxby, 2007; Schultz, Imamizu, Kawato, & Frith, 2004) (Figure 4). This finding shows that a distributed and interacting set of brain areas is likely to be involved in social processing. Second, activity in the most ventral part of the mPFC (the part closer to the belly rather than toward the top of the head), which has been most consistently associated with thinking about the self, was particularly active when subjects mentalized about people they rated as similar to themselves. Simulation is thought to be most likely for similar others, so this finding lends support to the conclusion that we use simulation to mentalize about others. After all, if you encounter someone who has the same musical taste as you, you will probably assume you have other things in common with him. By contrast, if you learn that someone loves music that you hate, you might expect him to differ from you in other ways (Srivastava, Guglielmo, & Beer, 2010). Using a simulation of our own feelings and thoughts will be most accurate if we have reason to think the person’s internal experiences are like our own. Thus, we may be most likely to use simulation to make inferences about others if we think they are similar to us.
This research is a good example of how social neuroscience is revealing the functional neuroanatomy of social behavior. That is, it tells us which brain areas are involved with social behavior. The mPFC (as well as other areas such as the STS, amygdala, and TPJ) is involved in making judgments about the self and others. This research also provides new information about how inferences are made about others. Whereas some have doubted the widespread use of simulation as a means for making inferences about others, the activation of the mPFC when mentalizing about others, and the sensitivity of this activation to similarity between self and other, provides evidence that simulation occurs.
What Is the Cost of Social Stress?
Stress is an unfortunately frequent experience for many of us. Stress – which can be broadly defined as a threat or challenge to our well-being – can result from everyday events like a course exam or more extreme events such as experiencing a natural disaster. When faced with a stressor, sympathetic nervous system activity increases in order to prepare our body to respond to the challenge. This produces what Selye (1950) called a fight or flight response. The release of hormones, which act as messengers from one part of an organism (e.g., a cell or gland) to another part of the organism, is part of the stress response.
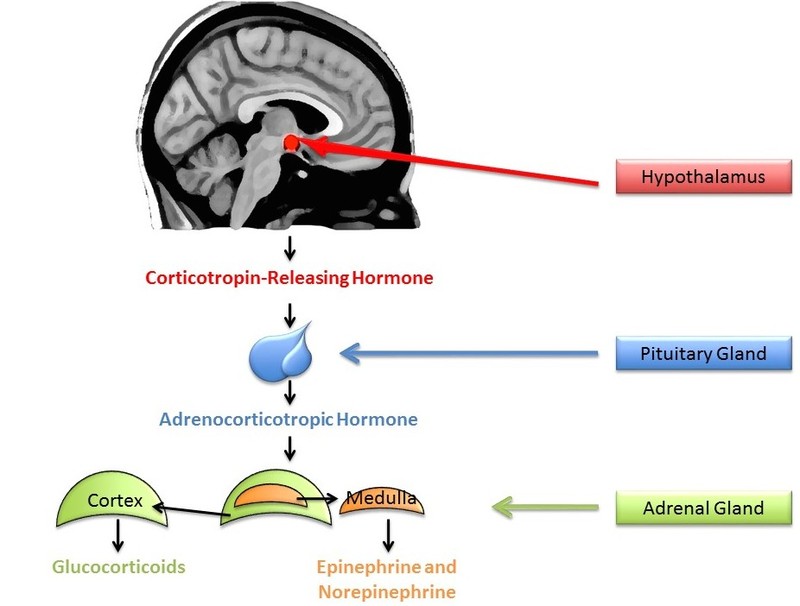
A small amount of stress can actually help us stay alert and active. In comparison, sustained stressors, or chronic stress, detrimentally affect our health and impair performance (Al’Absi, Hugdahl, & Lovallo, 2002; Black, 2002; Lazarus, 1974). This happens in part through the chronic secretion of stress-related hormones (e.g., Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Dickerson, Gable, Irwin, Aziz, & Kemeny, 2009). In particular, stress activates the hypothalamic-pituitary-adrenal (HPA) axis to release cortisol (see Figure 5 for a discussion). Chronic stress, by way of increases in cortisol, impairs attention, memory, and self-control (Arnsten, 2009). Cortisol levels can be measured non-invasively in bodily fluids, including blood and saliva. Researchers often collect a cortisol sample before and after a potentially stressful task. In one common collection method, subjects place polymer swabs under their tongue for 1 to 2 minutes to soak up saliva. The saliva samples are then stored and analyzed later to determine the level of cortisol present at each time point.
Whereas early stress researchers studied the effects of physical stressors like loud noises, social neuroscientists have been instrumental in studying how our interactions with other people can cause stress. This question has been addressed through neuroendocrinology, or the study of how the brain and hormones act in concert to coordinate the physiology of the body. One contribution of this work has been in understanding the conditions under which other people can cause stress. In one study, Dickerson, Mycek, and Zaldivar (2008) asked undergraduates to deliver a speech either alone or to two other people. When the students gave the speech in front of others, there was a marked increase in cortisol compared with when they were asked to give a speech alone. This suggests that like chronic physical stress, everyday social stressors, like having your performance judged by others, induces a stress response. Interestingly, simply giving a speech in the same room with someone who is doing something else did not induce a stress response. This suggests that the mere presence of others is not stressful, but rather it is the potential for them to judge us that induces stress.
Worrying about what other people think of us is not the only source of social stress in our lives. Other research has shown that interacting with people who belong to different social groups than us – what social psychologists call outgroup members – can increase physiological stress responses. For example, cardiovascular responses associated with stress like contractility of the heart ventricles and the amount of blood pumped by the heart (what is called cardiac output) are increased when interacting with outgroup as compared with ingroup members (i.e., people who belong to the same social group we do) (Mendes, Blascovich, Likel, & Hunter, 2002). This stress may derive from the expectation that interactions with dissimilar others will be uncomfortable (Stephan & Stephan, 1985) or concern about being judged as unfriendly and prejudiced if the interaction goes poorly (Plant & Devine, 2003).
The research just reviewed shows that events in our social lives can be stressful, but are social interactions always bad for us? No. In fact, while others can be the source of much stress, they are also a major buffer against stress. Research on social support shows that relying on a network of individuals in tough times gives us tools for dealing with stress and can ward off loneliness (Cacioppo & Patrick, 2008). For instance, people who report greater social support show a smaller increase in cortisol when performing a speech in front of two evaluators (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007).
What determines whether others will increase or decrease stress? What matters is the context of the social interaction. When it has potential to reflect badly on the self, social interaction can be stressful, but when it provides support and comfort, social interaction can protect us from the negative effects of stress. Using neuroendocrinology by measuring hormonal changes in the body has helped researchers better understand how social factors impact our body and ultimately our health.
Conclusions
Human beings are intensely social creatures – our lives are intertwined with other people and our health and well-being depend on others. Social neuroscience helps us to understand the critical function of how we make sense of and interact with other people. This module provides an introduction to what social neuroscience is and what we have already learned from it, but there is much still to understand. As we move forward, one exciting future direction will be to better understand how different parts of the brain and body interact to produce the numerous and complex patterns of social behavior that humans display. We hinted at some of this complexity when we reviewed research showing that while the mPFC is involved in mentalizing, other areas such as the STS, amygdala, and TPJ are as well. There are likely additional brain areas involved as well, interacting in ways we do not yet fully understand. These brain areas in turn control other aspects of the body to coordinate our responses during social interactions. Social neuroscience will continue to investigate these questions, revealing new information about how social processes occur, while also increasing our understanding of basic neural and physiological processes.
Outside Resources
- Society for Social Neuroscience
- http://www.s4sn.org
- Video: See a demonstration of fMRI data being collected.
- Video: See an example of EEG data being collected.
- Video: View two tasks frequently used in the lab to create stress – giving a speech in front of strangers, and doing math computations out loud in front of others. Notice how some subjects show obvious signs of stress, but in some situations, cortisol changes suggest that even people who appear calm are experiencing a physiological response associated with stress.
- Video: Watch a video used by Fritz Heider and Marianne Simmel in a landmark study on social perception published in 1944. Their goal was to investigate how we perceive other people, and they studied it by seeing how readily we apply people-like interpretations to non-social stimuli.
Discussion Questions
- Categorizing someone as a member of a social group can activate group stereotypes. EEG research suggests that social categorization occurs quickly and often automatically. What does this tell us about the likelihood of stereotyping occurring? How can we use this information to develop ways to stop stereotyping from happening?
- Watch this video, similar to what was used by Fritz Heider and Marianne Simmel in a landmark study on social perception published in 1944, and imagine telling a friend what happened in the video. http://intentionperception.org/wp-co...ider_Flash.swf. After watching the video, think about the following: Did you describe the motion of the objects solely in geometric terms (e.g., a large triangle moved from the left to the right), or did you describe the movements as actions of animate beings, maybe even of people (e.g., the circle goes into the house and shuts the door)? In the original research, 33 of 34 subjects described the action of the shapes using human terms. What does this tell us about our tendency to mentalize?
- Consider the types of things you find stressful. How many of them are social in nature (e.g., are related to your interactions with other people)? Why do you think our social relations have such potential for stress? In what ways can social relations be beneficial and serve as a buffer for stress?
Vocabulary
- Amygdala
- A region located deep within the brain in the medial area (toward the center) of the temporal lobes (parallel to the ears). If you could draw a line through your eye sloping toward the back of your head and another line between your two ears, the amygdala would be located at the intersection of these lines. The amygdala is involved in detecting relevant stimuli in our environment and has been implicated in emotional responses.
- Automatic process
- When a thought, feeling, or behavior occurs with little or no mental effort. Typically, automatic processes are described as involuntary or spontaneous, often resulting from a great deal of practice or repetition.
- Cortisol
- A hormone made by the adrenal glands, within the cortex. Cortisol helps the body maintain blood pressure and immune function. Cortisol increases when the body is under stress.
- Electroencephalogram
- A measure of electrical activity generated by the brain’s neurons.
- Fight or flight response
- The physiological response that occurs in response to a perceived threat, preparing the body for actions needed to deal with the threat.
- Functional magnetic resonance imaging
- A measure of changes in the oxygenation of blood flow as areas in the brain become active.
- Functional neuroanatomy
- Classifying how regions within the nervous system relate to psychology and behavior.
- Hormones
- Chemicals released by cells in the brain or body that affect cells in other parts of the brain or body.
- Hypothalamic-pituitary-adrenal (HPA) axis
- A system that involves the hypothalamus (within the brain), the pituitary gland (within the brain), and the adrenal glands (at the top of the kidneys). This system helps maintain homeostasis (keeping the body’s systems within normal ranges) by regulating digestion, immune function, mood, temperature, and energy use. Through this, the HPA regulates the body’s response to stress and injury.
- Ingroup
- A social group to which an individual identifies or belongs.
- Lesions
- Damage or tissue abnormality due, for example, to an injury, surgery, or a vascular problem.
- Medial prefrontal cortex
- An area of the brain located in the middle of the frontal lobes (at the front of the head), active when people mentalize about the self and others.
- Mentalizing
- The act of representing the mental states of oneself and others. Mentalizing allows humans to interpret the intentions, beliefs, and emotional states of others.
- Neuroendocrinology
- The study of how the brain and hormones act in concert to coordinate the physiology of the body.
- Outgroup
- A social group to which an individual does not identify or belong.
- Simulation
- Imaginary or real imitation of other people’s behavior or feelings.
- The act of mentally classifying someone into a social group (e.g., as female, elderly, a librarian).
- A subjective feeling of psychological or physical comfort provided by family, friends, and others.
- Stereotypes
- The beliefs or attributes we associate with a specific social group. Stereotyping refers to the act of assuming that because someone is a member of a particular group, he or she possesses the group’s attributes. For example, stereotyping occurs when we assume someone is unemotional just because he is man, or particularly athletic just because she is African American.
- Stress
- A threat or challenge to our well-being. Stress can have both a psychological component, which consists of our subjective thoughts and feelings about being threatened or challenged, as well as a physiological component, which consists of our body’s response to the threat or challenge (see “fight or flight response”).
- Superior temporal sulcus
- The sulcus (a fissure in the surface of the brain) that separates the superior temporal gyrus from the middle temporal gyrus. Located in the temporal lobes (parallel to the ears), it is involved in perception of biological motion or the movement of animate objects.
- Sympathetic nervous system
- A branch of the autonomic nervous system that controls many of the body’s internal organs. Activity of the SNS generally mobilizes the body’s fight or flight response.
- Temporal parietal junction
- The area where the temporal lobes (parallel to the ears) and parieta lobes (at the top of the head toward the back) meet. This area is important in mentalizing and distinguishing between the self and others.
References
Al’Absi, M., Hugdahl, K., & Lovallo, W. (2002). Adrenocortical stress responses and altered working memory performance. Psychophysiology, 39(1), 95–99.
Arnsten, A. F. T. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nature Neuroscience Reviews, 10(6), 410–422.
Black, P. (2002). Stress and the inflammatory response: A review of neurogenic inflammation. *Brain, Behavior, & Immunity, 16*, 622–653.
Cacioppo, J. T., & Berntson, G. G. (1992). Social psychological contributions to the decade of the brain: Doctrine of multilevel analysis. American Psychologist, 47, 1019–1028.
Cacioppo, J. T., & Patrick, B. (2008). Loneliness: Human nature and the need for social connection. New York, NY: W. W. Norton & Company.
Carruthers, P. and Smith, P. (1996). Theories of Theories of Mind. New York, NY: Cambridge University Press.
Davidson, R. J., Pizzagalli, D., Nitschke, J. B., & Putnam, K. (2002). Depression: Perspectives from affective neuroscience. Annual Review of Psychology, 53, 545–574.
Dickerson, S. S., Gable, S. L., Irwin, M. R., Aziz, N., & Kemeny, M. E. (2009). Social-evaluative threat and proinflammatory cytokine regulation an experimental laboratory investigation. Psychological Science, 20, 1237–1244.
Dickerson, S. S., Mycek, P. J., & Zaldivar, F. (2008). Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology, 27(1), 116–121.
Eisenberger, N. I., Taylor, S. E., Gable, S. L., Hilmert, C. J., & Lieberman, M. D. (2007). Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage, 35(4), 1601–1612.
Gallese, V., & Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences, 2, 493–501.
Gobbini, M. I., & Haxby, J. V. (2007). Neural systems for recognition of familiar faces. Neuropsychologia, 45(1), 32–41.
Goldman, A. I. (2005). Imitation, mind reading, and simulation. In S. Hurley & N. Chater (Eds.), Perspectives on imitation: From neuroscience to social science (Vol. 2: Imitation, human development, and culture, pp. 79–93). Cambridge, MA: MIT Press.
Ito, T. A., & Urland, G. R. (2003). Race and gender on the brain: Electrocortical measures of attention to race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology, 85, 616–626.
Ito, T.A., & Urland, G.R. (2005). The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, and Behavioral Neuroscience, 5, 21–36.
Kelley, W. M., Macrae, C. N., Wyland, C. L., Caglar, S., Inati, S., & Heatherton, T. F. (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14, 785–794.
Lazarus, R. S., (1974). Psychological stress and coping in adaptation and illness. *International Journal of Psychiatry in Medicine, 5*, 321–333.
Mendes, W. B., Blascovich, J., Lickel, B., & Hunter, S. (2002). Challenge and threat during social interactions with White and Black men. Personality and Social Psychology Bulletin, 28, 939–952.
Mitchell, J. P., Neil Macrae, C., & Banaji, M. R. (2005). Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage, 26(1), 251–257.
Mouchetant-Rostaing, Y., & Giard, M. H. (2003). Electrophysiological correlates of age and gender perception on human faces. Journal of Cognitive Neuroscience, 15, 900–910.
Phelps, E. A., & LeDoux, J. E. (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48, 175.
Plant, E. A., & Devine, P. G. (2003). The antecedents and implications of interracial anxiety. *Personality and Social Psychology Bulletin, 29*, 790–801.
Schultz, J., Imamizu, H., Kawato, M., & Frith, C. D. (2004). Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. Journal of Cognitive Neuroscience, 16, 1695–1705.
Selye, H. (1950). The physiology and pathology of exposure to stress. Montreal: Acta Inc.
Srivastava, S., Guglielmo, S., & Beer, J. S. (2010). Perceiving others’ personalities: Examining the dimensionality, assumed similarity to the self, and stability of perceiver effects. Journal of Personality and Social Psychology, 98, 520.
Stephan, W. G., & Stephan, C. W. (1985). Intergroup anxiety. Journal of Social Issues, 41(3), 157–175.
Whalen, P. J., & Phelps, E. A. (2009). The human amygdala. New York, NY: The Guilford Press.
Attributions
Adapted by Kenneth A. Koenigshofer, PhD, from Ito, T. A. & Kubota, J. T. (2021). Social neuroscience. In R. Biswas-Diener & E. Diener (Eds), Noba textbook series: Psychology. Champaign, IL: DEF publishers. Retrieved from http://noba.to/qyekc5gf
Authors

- Tiffany A. Ito is a Professor of Psychology and Neuroscience at the University of Colorado Boulder. Her research integrates neuroscience methods and theories to better understand social processes, with a particular focus on aspects of stereotyping and prejudice.
- Jennifer Kubota is an assistant professor at University of Delaware. She received her Ph.D. in social neuroscience from The University of Colorado Boulder. Her work focuses on the psychological and neural substrates of impression formation and their relation to decision-making.
Creative Commons License



 Social Neuroscience by Tiffany A. Ito and Jennifer T. Kubota is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.
Social Neuroscience by Tiffany A. Ito and Jennifer T. Kubota is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.
Minor editing of this content by Kenneth A. Koenigshofer, PhD. Chaffey College.
Theory of Mind and Social Cognition
Overview
We now continue exploration of social cognition by examining an important cognitive ability in humans essential for successful social interaction--theory of mind, a capacity that some scholars prefer to label “mentalizing” or “mindreading,” the ability to perceive and interpret other people’s behavior in terms of their mental states. The capacity may have evolved sometime in the last few million years. Theory of mind is thought to be a prerequisite for natural language acquisition, strategic social interaction, reflexive thought, and moral judgment. Humans need to understand minds in order to engage in the kinds of complex interactions that social communities (small and large) require--interactions that have given rise to the complex products of human cultural evolution. The capacity is severely limited in autism.
Theory of Mind
Brown University
One of the most remarkable human capacities is to perceive and understand mental states. This capacity, often labeled “theory of mind,” consists of an array of psychological processes that play essential roles in human social life. We review some of these roles, examine what happens when the capacity is deficient, and explore the many processes that make up the capacity to understand minds.
Learning Objectives
- Explain what theory of mind is.
- Enumerate the many domains of social life in which theory of mind is critical.
- Describe some characteristics of how autistic individuals differ in their processing of others’ minds.
- Describe and explain some of the many concepts and processes that comprise the human understanding of minds.
- Have a basic understanding of how ordinary people explain unintentional and intentional behavior.
Introduction
One of the most fascinating human capacities is the ability to perceive and interpret other people’s behavior in terms of their mental states. Having an appreciation for the workings of another person’s mind is considered a prerequisite for natural language acquisition (Baldwin & Tomasello, 1998), strategic social interaction (Zhang, Hedden, & Chia, 2012), reflexive thought (Bogdan, 2000), and moral judgment (Guglielmo, Monroe, & Malle, 2009). This capacity develops from early beginnings in the first year of life to the adult’s fast and often effortless understanding of others’ thoughts, feelings, and intentions. And though we must speculate about its evolutionary origin, we do have indications that the capacity evolved sometime in the last few million years.
In this module we will focus on two questions: What is the role of understanding others’ minds in human social life? And what is known about the mental processes that underlie such understanding? For simplicity, we will label this understanding “theory of mind,” even though it is not literally a “theory” that people have about the mind; rather, it is a capacity that some scholars prefer to label “mentalizing” or “mindreading.” But we will go behind all these labels by breaking down the capacity into distinct components: the specific concepts and mental processes that underlie the human understanding of minds.
First, let’s get clear about the roles that this understanding plays in social life.
The Role of Theory of Mind in Social Life

Put yourself in this scene: You observe two people’s movements, one behind a large wooden object, the other reaching behind him and then holding a thin object in front of the other. Without a theory of mind you would neither understand what this movement stream meant nor be able to predict either person’s likely responses. With the capacity to interpret certain physical movements in terms of mental states, perceivers can parse this complex scene into intentional actions of reaching and giving (Baird & Baldwin, 2001); they can interpret the actions as instances of offering and trading; and with an appropriate cultural script, they know that all that was going on was a customer pulling out her credit card with the intention to pay the cashier behind the register. People’s theory of mind thus frames and interprets perceptions of human behavior in a particular way—as perceptions of agents who can act intentionally and who have desires, beliefs, and other mental states that guide their actions (Perner, 1991; Wellman, 1990).
Not only would social perceivers without a theory of mind be utterly lost in a simple payment interaction; without a theory of mind, there would probably be no such things as cashiers, credit cards, and payment (Tomasello, 2003). Plain and simple, humans need to understand minds in order to engage in the kinds of complex interactions that social communities (small and large) require. And it is these complex social interactions that have given rise, in human cultural evolution, to houses, cities, and nations; to books, money, and computers; to education, law, and science.
The list of social interactions that rely deeply on theory of mind is long; here are a few highlights.
- Teaching another person new actions or rules by taking into account what the learner knows or doesn’t know and how one might best make him understand.
- Learning the words of a language by monitoring what other people attend to and are trying to do when they use certain words.
- Figuring out our social standing by trying to guess what others think and feel about us.
- Sharing experiences by telling a friend how much we liked a movie or by showing her something beautiful.
- Collaborating on a task by signaling to one another that we share a goal and understand and trust the other’s intention to pursue this joint goal.
Autism and Theory of Mind

Another way of appreciating the enormous impact that theory of mind has on social interactions is to study what happens when the capacity is severely limited, as in the case of autism (Tager-Flusberg, 2007). In a fascinating discussion in which (high-functioning) autistic individuals talk about their difficulties with other people’s minds (Blackburn, Gottschewski, George, & L—, 2000), one person reports: “I know people’s faces down to the acne scars on the left corners of their chins . . . and how the hairs of their eyebrows curl. . . . The best I can do is start picking up bits of data during my encounter with them because there’s not much else I can do. . . . I’m not sure what kind of information about them I’m attempting to process.” What seems to be missing, as another person with autism remarks, is an “automatic processing of ‘people information.’” Some autistic people report that they perceive others “in a more analytical way.” This analytical mode of processing, however, is very tiresome and slow: “Given time I may be able to analyze someone in various ways, and seem to get good results, but may not pick up on certain aspects of an interaction until I am obsessing over it hours or days later” (Blackburn et al., 2000).
So what is this magical potion that allows most people to gain quick and automatic access to other people’s minds and to recognize the meaning underlying human behavior? Scientific research has accumulated a good deal of knowledge in the past few decades, and here is a synopsis of what we know.
The Mental Processes Underlying Theory of Mind
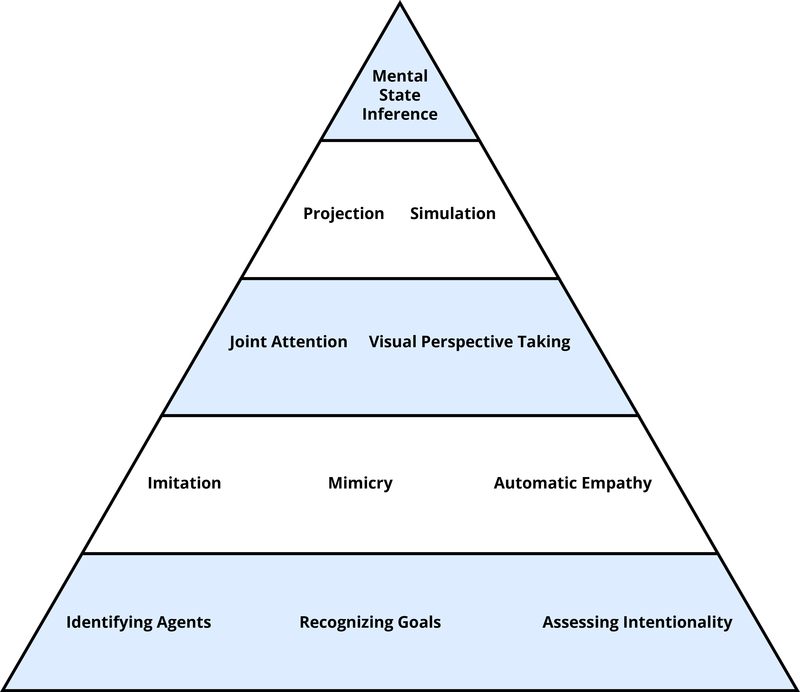
The first thing to note is that “theory of mind” is not a single thing. What underlies people’s capacity to recognize and understand mental states is a whole host of components—a toolbox, as it were, for many different but related tasks in the social world (Malle, 2008). Figure 1 shows some of the most important tools, organized in a way that reflects the complexity of involved processes: from simple and automatic on the bottom to complex and deliberate on the top. This organization also reflects development—from tools that infants master within the first 6–12 months to tools they need to acquire over the next 3–5 years. Strikingly, the organization also reflects evolution: monkeys have available the tools on the bottom; chimpanzees have available the tools at the second level; but only humans master the remaining tools above. Let’s look at a few of them in more detail.
Agents, Goals, and Intentionality
The agent category allows humans to identify those moving objects in the world that can act on their own. Features that even very young children take to be indicators of being an agent include being self-propelled, having eyes, and reacting systematically to the interaction partner’s behavior, such as following gaze or imitating (Johnson, 2000; Premack, 1990).
The process of recognizing goals builds on this agent category, because agents are characteristically directed toward goal objects, which means they seek out, track, and often physically contact said objects. Even before the end of their first year, infants recognize that humans reach toward an object they strive for even if that object changes location or if the path to the object contains obstacles (Gergely, Nádasdy, Csibra, & Bíró, 1995; Woodward, 1998). What it means to recognize goals, therefore, is to see the systematic and predictable relationship between a particular agent pursuing a particular object across various circumstances.
Through learning to recognize the many ways by which agents pursue goals, humans learn to pick out behaviors that are intentional. The concept of intentionality is more sophisticated than the goal concept. For one thing, human perceivers recognize that some behaviors can be unintentional even if they were goal-directed—such as when you unintentionally make a fool of yourself even though you had the earnest goal of impressing your date. To act intentionally you need, aside from a goal, the right kinds of beliefs about how to achieve the goal. Moreover, the adult concept of intentionality requires that an agent have the skill to perform the intentional action in question: If I am flipping a coin, trying to make it land on heads, and if I get it to land on heads on my first try, you would not judge my action of making it land on heads as intentional—you would say it was luck (Malle & Knobe, 1997).
Imitation, Synchrony, and Empathy

Imitation and empathy are two other basic capacities that aid the understanding of mind from childhood on (Meltzoff & Decety, 2003). Imitation is the human tendency to carefully observe others’ behaviors and do as they do—even if it is the first time the perceiver has seen this behavior. A subtle, automatic form of imitation is called mimicry, and when people mutually mimic one another they can reach a state of synchrony. Have you ever noticed when two people in conversation take on similar gestures, body positions, even tone of voice? They “synchronize” their behaviors by way of (largely) unconscious imitation. Such synchrony can happen even at very low levels, such as negative physiological arousal (Levenson & Ruef, 1992), though the famous claim of synchrony in women’s menstrual cycles is a myth (Yang & Schank, 2006). Interestingly, people who enjoy an interaction synchronize their behaviors more, and increased synchrony (even manipulated in an experiment) makes people enjoy their interaction more (Chartrand & Bargh, 1999). Some research findings suggest that synchronizing is made possible by brain mechanisms that tightly link perceptual information with motor information (when I see you move your arm, my arm-moving program is activated). In monkeys, highly specialized so-called mirror neurons fire both when the monkey sees a certain action and when it performs that same action (Rizzolatti, Fogassi, & Gallese, 2001). In humans, however, things are a bit more complex. In many everyday settings, people perceive uncountable behaviors and fortunately don’t copy all of them (just consider walking in a crowd—hundreds of your mirror neurons would fire in a blaze of confusion). Human imitation and mirroring is selective, triggering primarily actions that are relevant to the perceiver’s current state or aim.
Automatic empathy builds on imitation and synchrony in a clever way. If Bill is sad and expresses this emotion in his face and body, and if Elena watches or interacts with Bill, then she will subtly imitate his dejected behavior and, through well-practiced associations of certain behaviors and emotions, she will feel a little sad as well (Sonnby-Borgström, Jönsson, & Svensson, 2003). Thus, she empathizes with him—whether she wants to or not. Try it yourself. Type “sad human faces” into your Internet search engine and select images from your results. Look at 20 photos and pay careful attention to what happens to your face and to your mood. Do you feel almost a “pull” of some of your facial muscles? Do you feel a tinge of melancholy?
Joint Attention, Visual Perspective Taking
Going beyond the automatic, humans are capable of actively engaging with other people’s mental states, such as when they enter into situations of joint attention—like Marissa and Noah, who are each looking at an object and are both aware that each of them is looking at the object. This sounds more complicated than it really is. Just point to an object when a 3-year old is around and notice how both the child and you check in with each other, ensuring that you are really jointly engaging with the object. Such shared engagement is critical for children to learn the meaning of objects—both their value (is it safe and rewarding to approach?) and the words that refer to them (what do you call this?). When I hold up my keyboard and show it to you, we are jointly attending to it, and if I then say it’s called “Tastatur” in German, you know that I am referring to the keyboard and not to the table on which it had been resting.
Another important capacity of engagement is visual perspective taking: You are sitting at a dinner table and advise another person on where the salt is—do you consider that it is to her left even though it is to your right? When we overcome our egocentric perspective this way, we imaginatively adopt the other person’s spatial viewpoint and determine how the world looks from their perspective. In fact, there is evidence that we mentally “rotate” toward the other’s spatial location, because the farther away the person sits (e.g., 60, 90, or 120 degrees away from you) the longer it takes to adopt the person’s perspective (Michelon & Zacks, 2006).
Projection, Simulation (and the Specter of Egocentrism)
When imagining what it might be like to be in another person’s psychological position, humans have to go beyond mental rotation. One tool to understand the other’s thoughts or feelings is simulation—using one’s own mental states as a model for others’ mental states: “What would it feel like sitting across from the stern interrogator? I would feel scared . . .” An even simpler form of such modeling is the assumption that the other thinks, feels, wants what we do—which has been called the “like-me” assumption (Meltzoff, 2007) or the inclination toward social projection (Krueger, 2007). In a sense, this is an absence of perspective taking, because we assume that the other’s perspective equals our own. This can be an effective strategy if we share with the other person the same environment, background, knowledge, and goals, but it gets us into trouble when this presumed common ground is in reality lacking. Let’s say you know that Brianna doesn’t like Fred’s new curtains, but you hear her exclaim to Fred, “These are beautiful!” Now you have to predict whether Fred can figure out that Brianna was being sarcastic. It turns out that you will have a hard time suppressing your own knowledge in this case and you may overestimate how easy it is for Fred to spot the sarcasm (Keysar, 1994). Similarly, you will overestimate how visible that pimple is on your chin—even though it feels big and ugly to you, in reality very few people will ever notice it (Gilovich & Savitsky, 1999). So the next time when you spot a magnificent bird high up in the tree and you get impatient with your friend who just can’t see what is clearly obvious, remember: it’s obvious to you.
What all these examples show is that people use their own current state—of knowledge, concern, or perception—to grasp other people’s mental states. And though they often do so correctly, they also get things wrong at times. This is why couples counselors, political advisors, and Buddhists agree on at least one thing: we all need to try harder to recognize our egocentrism and actively take other people’s perspective—that is, grasp their actual mental states, even if (or especially when) they are different from our own.
Explicit Mental State Inference
The ability to truly take another person’s perspective requires that we separate what we want, feel, and know from what the other person is likely to want, feel, and know. To do so humans make use of a variety of information. For one thing, they rely on stored knowledge—both general knowledge (“Everybody would be nervous when threatened by a man with a gun”) and agent-specific knowledge (“Joe was fearless because he was trained in martial arts”). For another, they critically rely on perceived facts of the concrete situation—such as what is happening to the agent, the agent’s facial expressions and behaviors, and what the person saw or didn’t see.
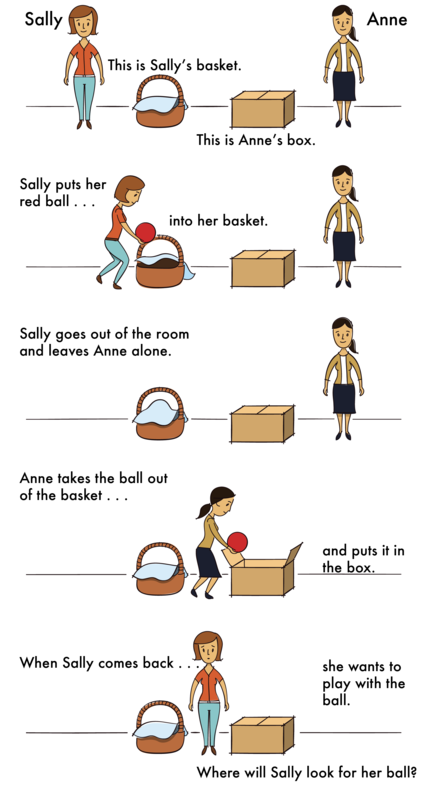
This capacity of integrating multiple lines of information into a mental-state inference develops steadily within the first few years of life, and this process has led to a substantial body of research (Wellman, Cross, & Watson, 2001). The research began with a clever experiment by Wimmer and Perner (1983), who tested whether children can pass a false-belief test (see Figure 2). The child is shown a picture story of Sally, who puts her ball in a basket and leaves the room. While Sally is out of the room, Anne comes along and takes the ball from the basket and puts it inside a box. The child is then asked where Sally thinks the ball is located when she comes back to the room. Is she going to look first in the box or in the basket?
The right answer is that she will look in the basket, because that’s where she put it and thinks it is; but we have to infer this false belief against our own better knowledge that the ball is in the box. This is very difficult for children before the age of 4, and it usually takes some cognitive effort in adults (Epley, Morewedge, & Keysar, 2004).
The challenge is clear: People are good at automatically relating to other people, using their own minds as a fitting model for others’ minds. But people need to recognize when to step out of their own perspective and truly represent the other person’s perspective—which may harbor very different thoughts, feelings, and intentions.
Tools in Summary
We have seen that the human understanding of other minds relies on many tools. People process such information as motion, faces, and gestures and categorize it into such concepts as agent, intentional action, or fear. They rely on relatively automatic psychological processes, such as imitation, joint attention, and projection. And they rely on more effortful processes, such as simulation and mental-state inference. These processes all link behavior that humans observe to mental states that humans infer. If we call this stunning capacity a “theory,” it is a theory of mind and behavior.
Folk Explanations of Behavior
Nowhere is this mind–behavior link clearer than in people’s explanations of behavior—when they try to understand why somebody acted or felt a certain way. People have a strong need to answer such “why” questions, from the trivial to the significant: why the neighbor’s teenage daughter is wearing a short skirt in the middle of winter; why the policeman is suddenly so friendly; why the murderer killed three people. The need to explain this last behavior seems puzzling, because typical benefits of explanation are absent: We do not need to predict or control the criminal’s behavior since we will never have anything to do with him. Nonetheless, we have an insatiable desire to understand, to find meaning in this person’s behavior—and in people’s behavior generally.
This makes evolutionary sense for at least two reasons: 1) because we are highly social creatures by virtue of our genetic evolution as a species, and success in the social group, including being valued by others in your group, was especially important to our survival in our evolutionary past (if you "don't get" others and consequently aren't valued and liked by the group, they are less likely to risk themselves to save you from a predator or to share food and other resources with you), strong motivation to understand other people would be favored by natural selection; 2) strong motivation to understand behavior of others increases chances of being able to predict behavior of others, and to predict their intentions, thereby allowing prediction of their future behavior allowing strategic preparation for it increasing your success in your social interactions, essential to human adaptation.

Older theories of how people explain and understand behavior suggested that people merely identify causes of the behavior (e.g., Kelley, 1967). That is true for most unintentional behaviors—tripping, having a headache, calling someone by the wrong name. But to explain intentional behaviors, people use a more sophisticated framework of interpretation, which follows directly from their concept of intentionality and the associated mental states they infer (Malle, 2004). We have already mentioned the complexity of people’s concept of intentionality; here it is in full (Malle & Knobe, 1997): For an agent to perform a behavior intentionally, she must have a desire for an outcome (what we had called a goal), beliefs about how a particular action leads to the outcome, and an intention to perform that action; if the agent then actually performs the action with awareness and skill, people take it to be an intentional action. To explain why the agent performed the action, humans try to make the inverse inference of what desire and what beliefs the agent had that led her to so act, and these inferred desires and beliefs are the reasons for which she acted. What was her reason for wearing a short skirt in the winter? “She wanted to annoy her mother.” What was the policeman’s reason for suddenly being so nice? “He thought he was speaking with an influential politician.” What was his reason for killing three people? In fact, with such extreme actions, people are often at a loss for an answer. If they do offer an answer, they frequently retreat to “causal history explanations” (Malle, 1999), which step outside the agent’s own reasoning and refer instead to more general background facts—for example, that he was mentally ill or a member of an extremist group. But people clearly prefer to explain others’ actions by referring to their beliefs and desires, the specific reasons for which they acted.
By relying on a theory of mind, explanations of behavior make meaningful what would otherwise be inexplicable motions—just like in our initial example of two persons passing some object between them. We recognize that the customer wanted to pay and that’s why she passed her credit card to the cashier, who in turn knew that he was given a credit card and swiped it. It all seems perfectly clear, almost trivial to us. But that is only because humans have a theory of mind and use it to retrieve the relevant knowledge, simulate the other people’s perspective, infer beliefs and desires, and explain what a given action means. Humans do this effortlessly and often accurately. Moreover, they do it within seconds or less. What’s so special about that? Well, it takes years for a child to develop this capacity, and it took our species a few million years to evolve it. That’s pretty special.
Outside Resources
- Blog: On the debate about menstrual synchrony
- http://blogs.scientificamerican.com/context-and-variation/2011/11/16/menstrual-synchrony/
- Blog: On the debates over mirror neurons
- http://blogs.scientificamerican.com/guest-blog/2012/11/06/whats-so-special-about-mirror-neurons/
- Book: First and last chapters of Zunshine, L. (2006). Why we read fiction: Theory of mind and the novel. Columbus, OH: Ohio State University Press.
- https://ohiostatepress.org/Books/Book PDFs/Zunshine Why.pdf
- Movie: A movie that portrays the social difficulties of a person with autism: Adam (Fox Searchlight Pictures, 2009)
- http://www.imdb.com/title/tt1185836/?ref_=fn_tt_tt_1
- ToM and Autism TEDx Talks
- https://www.ted.com/playlists/153/the_autism_spectrum
- Video: TED talk on autism
- http://www.ted.com/talks/temple_grandin_the_world_needs_all_kinds_of_minds.html
- Video: TED talk on empathy
- http://blog.ted.com/2011/04/18/a-radical-experiment-in-empathy-sam-richards-at-ted-com/
- Video: TED talk on theory of mind and moral judgment
- http://www.ted.com/talks/rebecca_saxe_how_brains_make_moral_judgments.html
- Video: Test used by Baron Cohen (prior to the core study) to investigate whether autistic children had a theory of mind by using a false belief task.
- Video: Theory of mind development
Discussion Questions
- Recall a situation in which you tried to infer what a person was thinking or feeling but you just couldn’t figure it out, and recall another situation in which you tried the same but succeeded. Which tools were you able to use in the successful case that you didn’t or couldn’t use in the failed case?
- Mindfulness training improves keen awareness of one’s own mental states. Look up a few such training programs (easily found online) and develop a similar training program to improve awareness of other people’s minds.
- In the near future we will have robots that closely interact with people. Which theory of mind tools should a robot definitely have? Which ones are less important? Why?
- Humans assume that everybody has the capacity to make choices and perform intentional actions. But in a sense, a choice is just a series of brain states, caused by previous brain states and states of the world, all governed by the physical laws of the universe. Is the concept of choice an illusion?
- The capacity to understand others’ minds is intimately related to another unique human capacity: language. How might these two capacities have evolved? Together? One before the other? Which one?
Vocabulary
- Automatic empathy
- A social perceiver unwittingly taking on the internal state of another person, usually because of mimicking the person’s expressive behavior and thereby feeling the expressed emotion.
- False-belief test
- An experimental procedure that assesses whether a perceiver recognizes that another person has a false belief—a belief that contradicts reality.
- Folk explanations of behavior
- People’s natural explanations for why somebody did something, felt something, etc. (differing substantially for unintentional and intentional behaviors).
- Intention
- An agent’s mental state of committing to perform an action that the agent believes will bring about a desired outcome.
- Intentionality
- The quality of an agent’s performing a behavior intentionally—that is, with skill and awareness and executing an intention (which is in turn based on a desire and relevant beliefs).
- Joint attention
- Two people attending to the same object and being aware that they both are attending to it.
- Mimicry
- Copying others’ behavior, usually without awareness.
- Mirror neurons
- Neurons identified in monkey brains that fire both when the monkey performs a certain action and when it perceives another agent performing that action.
- Projection
- A social perceiver’s assumption that the other person wants, knows, or feels the same as the perceiver wants, know, or feels.
- Simulation
- The process of representing the other person’s mental state.
- Synchrony
- Two people displaying the same behaviors or having the same internal states (typically because of mutual mimicry).
- Theory of mind
- The human capacity to understand minds, a capacity that is made up of a collection of concepts (e.g., agent, intentionality) and processes (e.g., goal detection, imitation, empathy, perspective taking).
- Visual perspective taking
- Can refer to visual perspective taking (perceiving something from another person’s spatial vantage point) or more generally to effortful mental state inference (trying to infer the other person’s thoughts, desires, emotions).
References
Baird, J. A., & Baldwin, D. A. (2001). Making sense of human behavior: Action parsing and intentional inference. In B. F. Malle, L. J. Moses, & D. A. Baldwin (Eds.), Intentions and intentionality: Foundations of social cognition (pp. 193–206). Cambridge, MA:MIT Press.
Baldwin, D. A., & Tomasello, M. (1998). Word learning: A window on early pragmatic understanding. In E. V. Clark (Ed.), The proceedings of the twenty-ninth annual child language research forum (pp. 3–23). Chicago, IL: Center for the Study of Language and Information.
Blackburn, J., Gottschewski, K., George, E., & L—, N. (2000, May). A discussion about theory of mind: From an autistic perspective. Proceedings of Autism Europe’s 6th International Congress. Glasgow. Retrieved from http://archive.autistics.org/library/AE2000-ToM.html.
Bogdan, R. (2000). Minding minds: Evolving a reflexing mind by interpreting others. Cambridge, MA: MIT Press.
Chartrand, T. L., & Bargh, J. A. (1999). The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology, 76, 893–910.
Epley, N., Morewedge, C. K., & Keysar, B. (2004). Perspective taking in children and adults: Equivalent egocentrism but differential correction. Journal of Experimental Social Psychology, 40, 760–768.
Gergely, G., Nádasdy, Z., Csibra, G., & Bíró, S. (1995). Taking the intentional stance at 12 months of age. Cognition, 56, 165–193.
Gilovich, T., & Savitsky, K. (1999). The spotlight effect and the illusion of transparency: Egocentric assessments of how we are seen by others. Current Directions in Psychological Science, 8, 165–168.
Guglielmo, S., Monroe, A. E., & Malle, B. F. (2009). At the heart of morality lies folk psychology. Inquiry: An Interdisciplinary Journal of Philosophy, 52, 449–466.
Johnson, S. C. (2000). The recognition of mentalistic agents in infancy. Trends in Cognitive Sciences, 4, 22–28.
Kelley, H. H. (1967). Attribution theory in social psychology. In D. Levine (Ed.), Nebraska Symposium on Motivation (Vol. 15, pp. 192–240). Lincoln: University of Nebraska Press.
Keysar, B. (1994). The illusory transparency of intention: Linguistic perspective taking in text. Cognitive Psychology, 26, 165–208.
Krueger, J. I. (2007). From social projection to social behaviour. European Review of Social Psychology, 18, 1–35.
Levenson, R. W., & Ruef, A. M. (1992). Empathy: A physiological substrate. Journal of Personality and Social Psychology, 63, 234–246.
Malle, B. F. (2008). The fundamental tools, and possibly universals, of social cognition. In R. M. Sorrentino & S. Yamaguchi (Eds.), Handbook of motivation and cognition across cultures (pp. 267–296). New York, NY: Elsevier/Academic Press.
Malle, B. F. (2004). How the mind explains behavior: Folk explanations, meaning, and social interaction. Cambridge, MA: MIT Press.
Malle, B. F. (1999). How people explain behavior: A new theoretical framework. Personality and Social Psychology Review, 3, 23–48.
Malle, B. F., & Knobe, J. (1997). The folk concept of intentionality. Journal of Experimental Social Psychology, 33, 101–121.
Meltzoff, A. N. (2007). “Like me”: A foundation for social cognition. Developmental Science, 10, 126–134.
Meltzoff, A. N., & Decety, J. (2003). What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358, 491–500.
Michelon, P., & Zacks, J. M. (2006). Two kinds of visual perspective taking. Perception & Psychophysics, 68, 327–337.
Perner, J. (1991). Understanding the representational mind. Cambridge, MA: MIT Press.
Premack, D. (1990). The infant’s theory of self-propelled objects. Cognition, 36, 1–16.
Rizzolatti, G., Fogassi, L., & Gallese, V. (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience, 2, 661–670.
Sonnby-Borgström, M., Jönsson, P., & Svensson, O. (2003). Emotional empathy as related to mimicry reactions at different levels of information processing. Journal of Nonverbal Behavior, 27, 3–23.
Tager-Flusberg, H. (2007). Evaluating the theory-of-mind hypothesis of autism. Current Directions in Psychological Science, 16, 311–315.
Tomasello, M. (2003). The key is social cognition. In D. Gentner & S. Goldin-Meadow (Eds.), Language in mind: Advances in the study of language and thought (pp. 47–57). Cambridge, MA: MIT Press.
Wellman, H. M. (1990). The child’s theory of mind. Cambridge, MA: MIT Press.
Wellman, H. M., Cross, D., & Watson, J. (2001). Meta-analysis of theory-of-mind development: The truth about false belief. Child Development, 72, 655–684.
Wimmer, H., & Perner, J. (1983). Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition, 13, 103–128.
Woodward, A. L. (1998). Infants selectively encode the goal object of an actor’s reach. Cognition, 69, 1–34.
Yang, Z., & Schank, J. C. (2006). Women do not synchronize their menstrual cycles. Human Nature, 17, 433–447.
Zhang, J., Hedden, T., & Chia, A. (2012). Perspective-taking and depth of theory-of-mind reasoning in sequential-move games. Cognitive Science, 36, 560–573.
Attributions
Adapted by Kenneth A. Koenigshofer, Ph.D. from Malle, B. (2021). Theory of mind. In R. Biswas-Diener & E. Diener (Eds), Noba textbook series: Psychology. Champaign, IL: DEF publishers. Retrieved from http://noba.to/a8wpytg3
Authors

- Bertram Malle, Professor at Brown University, was trained in psychology, philosophy, and linguistics in Austria and at Stanford University. He received an SESP Dissertation award, an NSF CAREER award, and was president of the Society of Philosophy and Psychology. His research focuses on social cognition, moral judgment, and more recently human-robot interaction.
Creative Commons License



 Theory of Mind by Bertram Malle is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.
Theory of Mind by Bertram Malle is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.
Paragraph on evolutionary rationale for motivation to understand others in "Folk Explanations of Behavior" added by Kenneth A. Koenigshofer, PhD. Chaffey College.
Disorders of the Social Brain
Overview
We now continue exploration of social cognition by examining a spectrum of disorders of social processing and the brain mechanisms involved. Here we encounter social neuroscience once again. Autism is in the category of pervasive developmental disorders, which includes Asperger's disorder, childhood disintegrative disorder, autistic disorder, and pervasive developmental disorder - not otherwise specified. These disorders, together, are labeled autism spectrum disorder (ASD). ASD is defined by the presence of profound difficulties in social interactions and communication combined with the presence of repetitive or restricted interests, cognition and behaviors. The social brain is a set of interconnected neuroanatomical structures that process social information, enabling the recognition of other individuals and the evaluation their mental states. The social brain is hypothesized to consist of the amygdala, the orbital frontal cortex (OFC), fusiform gyrus (FG), and the posterior superior temporal sulcus (STS) region, among other structures. Because autism is a developmental disorder, it is particularly important to diagnose and treat ASD early in life.
People with autism spectrum disorder (ASD) suffer from a profound social disability. Social neuroscience is the study of the parts of the brain that support social interactions or the “social brain.” This module provides an overview of ASD and focuses on understanding how social brain dysfunction leads to ASD. Our increasing understanding of the social brain and its dysfunction in ASD will allow us to better identify the genes that cause ASD and will help us to create and pick out treatments to better match individuals. Because social brain systems emerge in infancy, social neuroscience can help us to figure out how to diagnose ASD even before the symptoms of ASD are clearly present. This is a hopeful time because social brain systems remain malleable well into adulthood and thus open to creative new interventions that are informed by state-of-the-art science.
Learning Objectives
- Know the basic symptoms of ASD.
- Distinguish components of the social brain and understand their dysfunction in ASD.
- Appreciate how social neuroscience may facilitate the diagnosis and treatment of ASD.
Defining Autism Spectrum Disorder
Autism Spectrum Disorder (ASD) is a developmental disorder that usually emerges in the first three years and persists throughout the individual’s life. Though the key symptoms of ASD fall into three general categories (see below), each person with ASD exhibits symptoms in these domains in different ways and to varying degrees. This phenotypic heterogeneity reflects the high degree of variability in the genes underlying ASD (Geschwind & Levitt, 2007). Though we have identified genetic differences associated with individual cases of ASD, each accounts for only a small number of the actual cases, suggesting that no single genetic cause will apply in the majority of people with ASD. There is currently no biological test for ASD.
Autism is in the category of pervasive developmental disorders, which includes Asperger's disorder, childhood disintegrative disorder, autistic disorder, and pervasive developmental disorder - not otherwise specified. These disorders, together, are labeled autism spectrum disorder (ASD). ASD is defined by the presence of profound difficulties in social interactions and communication combined with the presence of repetitive or restricted interests, cognitions and behaviors. The diagnostic process involves a combination of parental report and clinical observation. Children with significant impairments across the social/communication domain who also exhibit repetitive behaviors can qualify for the ASD diagnosis. There is wide variability in the precise symptom profile an individual may exhibit.
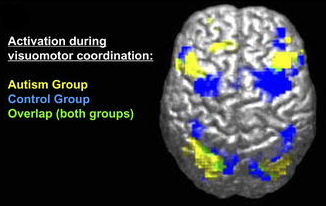
Since Kanner first described ASD in 1943, important commonalities in symptom presentation have been used to compile criteria for the diagnosis of ASD. These diagnostic criteria have evolved during the past 70 years and continue to evolve (e.g., see the recent changes to the diagnostic criteria on the American Psychiatric Association’s website, http://www.dsm5.org/), yet impaired social functioning remains a required symptom for an ASD diagnosis. Deficits in social functioning are present in varying degrees for simple behaviors such as eye contact, and complex behaviors like navigating the give and take of a group conversation for individuals of all functioning levels (i.e. high or low IQ). Moreover, difficulties with social information processing occur in both visual (e.g., Pelphrey et al., 2002) and auditory (e.g., Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998) sensory modalities.
Consider the results of an eye tracking study in which Pelphrey and colleagues (2002) observed that individuals with autism did not make use of the eyes when judging facial expressions of emotion (see right panels of Figure 1). While repetitive behaviors or language deficits are seen in other disorders (e.g., obsessive-compulsive disorder and specific language impairment, respectively), basic social deficits of this nature are unique to ASD. Onset of the social deficits appears to precede difficulties in other domains (Osterling, Dawson, & Munson, 2002) and may emerge as early as 6 months of age (Maestro et al., 2002).
Defining the Social Brain
Within the past few decades, research has elucidated specific brain circuits that support perception of humans and other species. This social perception refers to “the initial stages in the processing of information that culminates in the accurate analysis of the dispositions and intentions of other individuals” (Allison, Puce, & McCarthy, 2000). Basic social perception is a critical building block for more sophisticated social behaviors, such as thinking about the motives and emotions of others. Brothers (1990) first suggested the notion of a social brain, a set of interconnected neuroanatomical structures that process social information, enabling the recognition of other individuals and the evaluation their mental states (e.g., intentions, dispositions, desires, and beliefs).
The social brain is hypothesized to consist of the amygdala, the orbital frontal cortex (OFC), fusiform gyrus (FG), and the posterior superior temporal sulcus (STS) region, among other structures. Though all areas work in coordination to support social processing, each appears to serve a distinct role. The amygdala helps us recognize the emotional states of others (e.g., Morris et al., 1996) and also to experience and regulate our own emotions (e.g., LeDoux, 1992). The OFC supports the "reward" feelings we have when we are around other people (e.g., Rolls, 2000). The FG, located at the bottom of the surface of the temporal lobes detects faces and supports face recognition (e.g., Puce, Allison, Asgari, Gore, & McCarthy, 1996). The posterior STS region recognizes the biological motion, including eye, hand and other body movements, and helps to interpret and predict the actions and intentions of others (e.g., Pelphrey, Morris, Michelich, Allison, & McCarthy, 2005).
Current Understanding of Social Perception in ASD
The social brain is of great research interest because the social difficulties characteristic of ASD are thought to relate closely to the functioning of this brain network. Functional magnetic resonance imaging (fMRI) and event-related potentials (ERP) are complementary brain imaging methods used to study activity in the brain across the lifespan. Each method measures a distinct facet of brain activity and contributes unique information to our understanding of brain function.
FMRI uses powerful magnets to measure the levels of oxygen within the brain, which vary according to changes in neural activity. As the neurons in specific brain regions “work harder”, they require more oxygen. FMRI detects the brain regions that exhibit a relative increase in blood flow (and oxygen levels) while people listen to or view social stimuli in the MRI scanner. The areas of the brain most crucial for different social processes are thus identified, with spatial information being accurate to the millimeter.
In contrast, ERP provides direct measurements of the firing of groups of neurons in the cortex. Non-invasive sensors on the scalp record the small electrical currents created by this neuronal activity while the subject views stimuli or listens to specific kinds of information. While fMRI provides information about where brain activity occurs, ERP specifies when by detailing the timing of processing at the millisecond pace at which it unfolds.
ERP and fMRI are complementary, with fMRI providing excellent spatial resolution and ERP offering outstanding temporal resolution. Together, this information is critical to understanding the nature of social perception in ASD. To date, the most thoroughly investigated areas of the social brain in ASD are the superior temporal sulcus (STS), which underlies the perception and interpretation of biological motion, and the fusiform gyrus (FG), which supports face perception. Heightened sensitivity to biological motion (for humans, motion such as walking) serves an essential role in the development of humans and other highly social species. Emerging in the first days of life, the ability to detect biological motion helps to orient vulnerable young to critical sources of sustenance, support, and learning, and develops independent of visual experience with biological motion (e.g., Simion, Regolin, & Bulf, 2008). This inborn “life detector” serves as a foundation for the subsequent development of more complex social behaviors (Johnson, 2006).

From very early in life, children with ASD display reduced sensitivity to biological motion (Klin, Lin, Gorrindo, Ramsay, & Jones, 2009). Individuals with ASD have reduced activity in the STS during biological motion perception. Similarly, people at increased genetic risk for ASD but who do not develop symptoms of the disorder (i.e. unaffected siblings of individuals with ASD) show increased activity in this region, which is hypothesized to be a compensatory mechanism to offset genetic vulnerability (Kaiser et al., 2010).
In typical development, preferential attention to faces and the ability to recognize individual faces emerge in the first days of life (e.g., Goren, Sarty, & Wu, 1975). The special way in which the brain responds to faces usually emerges by three months of age (e.g., de Haan, Johnson, & Halit, 2003) and continues throughout the lifespan (e.g., Bentin et al., 1996). Children with ASD, however, tend to show decreased attention to human faces by six to 12 months (Osterling & Dawson, 1994). Children with ASD also show reduced activity in the FG when viewing faces (e.g., Schultz et al., 2000). Slowed processing of faces (McPartland, Dawson, Webb, Panagiotides, & Carver, 2004) is a characteristic of people with ASD that is shared by parents of children with ASD (Dawson, Webb, & McPartland, 2005) and infants at increased risk for developing ASD because of having a sibling with ASD (McCleery, Akshoomoff, Dobkins, & Carver, 2009). Behavioral and attentional differences in face perception and recognition are evident in children and adults with ASD as well (e.g., Hobson, 1986).
Exploring Diversity in ASD
Because of the limited quality of the behavioral methods used to diagnose ASD and current clinical diagnostic practice, which permits similar diagnoses despite distinct symptom profiles (McPartland, Webb, Keehn, & Dawson, 2011), it is possible that the group of children currently referred to as having ASD may actually represent different syndromes with distinct causes. Examination of the social brain may well reveal diagnostically meaningful subgroups of children with ASD. Measurements of the “where” and “when” of brain activity during social processing tasks provide reliable sources of the detailed information needed to profile children with ASD with greater accuracy. These profiles, in turn, may help to inform treatment of ASD by helping us to match specific treatments to specific profiles.
The integration of imaging methods is critical for this endeavor. Using face perception as an example, the combination of fMRI and ERP could identify who, of those individuals with ASD, shows anomalies in the FG and then determine the stage of information processing at which these impairments occur. Because different processing stages often reflect discrete cognitive processes, this level of understanding could encourage treatments that address specific processing deficits at the neural level.
For example, differences observed in the early processing stages might reflect problems with low-level visual perception, while later differences would indicate problems with higher-order processes, such as emotion recognition. These same principles can be applied to the broader network of social brain regions and, combined with measures of behavioral functioning, could offer a comprehensive profile of brain-behavior performance for a given individual. A fundamental goal for this kind of subgroup approach is to improve the ability to tailor treatments to the individual.
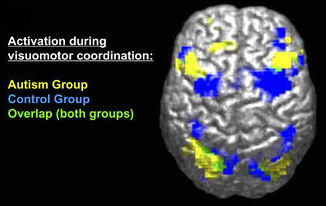
Another objective is to improve the power of other scientific tools. Most studies of individuals with ASD compare groups of individuals, for example, individuals on with ASD compared to typically developing peers. However, studies have also attempted to compare children across the autism spectrum by group according to differential diagnosis (e.g., Asperger’s disorder versus autistic disorder), or by other behavioral or cognitive characteristics (e.g., cognitively able versus intellectually disabled or anxious versus non-anxious). Yet, the power of a scientific study to detect these kinds of significant, meaningful, individual differences is only as strong as the accuracy of the factor used to define the compared groups.
The identification of distinct subgroups within the autism spectrum according to information about the brain would allow for a more accurate and detailed exposition of the individual differences seen in those with ASD. This is especially critical for the success of investigations into the genetic basis of ASD. As mentioned before, the genes discovered thus far account for only a small portion of ASD cases. If meaningful, quantitative distinctions in individuals with ASD are identified; a more focused examination into the genetic causes specific to each subgroup could then be pursued. Moreover, distinct findings from neuroimaging, or biomarkers, can help guide genetic research. Endophenotypes, or characteristics that are not immediately available to observation but that reflect an underlying genetic liability for disease, expose the most basic components of a complex psychiatric disorder and are more stable across the lifespan than observable behavior (Gottesman & Shields, 1973). By describing the key characteristics of ASD in these objective ways, neuroimaging research will facilitate identification of genetic contributions to ASD.
Atypical Brain Development Before the Emergence of Atypical Behavior
Because autism is a developmental disorder, it is particularly important to diagnose and treat ASD early in life. Early deficits in attention to biological motion, for instance, derail subsequent experiences in attending to higher level social information, thereby driving development toward more severe dysfunction and stimulating deficits in additional domains of functioning, such as language development. The lack of reliable predictors of the condition during the first year of life has been a major impediment to the effective treatment of ASD. Without early predictors, and in the absence of a firm diagnosis until behavioral symptoms emerge, treatment is often delayed for two or more years, eclipsing a crucial period in which intervention may be particularly successful in ameliorating some of the social and communicative impairments seen in ASD.
In response to the great need for sensitive (able to identify subtle cases) and specific (able to distinguish autism from other disorders) early indicators of ASD, such as biomarkers, many research groups from around the world have been studying patterns of infant development using prospective longitudinal studies of infant siblings of children with ASD and a comparison group of infant siblings without familial risks. Such designs gather longitudinal information about developmental trajectories across the first three years of life for both groups followed by clinical diagnosis at approximately 36 months.

These studies are problematic in that many of the social features of autism do not emerge in typical development until after 12 months of age, and it is not certain that these symptoms will manifest during the limited periods of observation involved in clinical evaluations or in pediatricians’ offices. Moreover, across development, but especially during infancy, behavior is widely variable and often unreliable, and at present, behavioral observation is the only means to detect symptoms of ASD and to confirm a diagnosis. This is quite problematic because, even highly sophisticated behavioral methods, such as eye tracking (see Figure 1), do not necessarily reveal reliable differences in infants with ASD (Ozonoff et al., 2010). However, measuring the brain activity associated with social perception can detect differences that do not appear in behavior until much later. The identification of biomarkers utilizing the imaging methods we have described offers promise for earlier detection of atypical social development.
ERP measures of brain response predict subsequent development of autism in infants as young as six months old who showed normal patterns of visual fixation (as measured by eye tracking) (Elsabbagh et al., 2012). This suggests the great promise of brain imaging for earlier recognition of ASD. With earlier detection, treatments could move from addressing existing symptoms to preventing their emergence by altering the course of abnormal brain development and steering it toward normality.
Hope for Improved Outcomes
The brain imaging research described above offers hope for the future of ASD treatment. Many of the functions of the social brain demonstrate significant plasticity, meaning that their functioning can be affected by experience over time. In contrast to theories that suggest difficulty processing complex information or communicating across large expanses of cortex (Minshew & Williams, 2007), this malleability of the social brain is a positive prognosticator for the development of treatment. The brains of people with ASD are not wired to process optimally social information. But this does not mean that these systems are irretrievably broken. Given the observed plasticity of the social brain, remediation of these difficulties may be possible with appropriate and timely intervention.
Outside Resources
- Web: American Psychiatric Association’s website for the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders
- http://www.dsm5.org
- Web: Autism Science Foundation - organization supporting autism research by providing funding and other assistance to scientists and organizations conducting, facilitating, publicizing and disseminating autism research. The organization also provides information about autism to the general public and serves to increase awareness of autism spectrum disorders and the needs of individuals and families affected by autism.
- http://www.autismsciencefoundation.org/
- Web: Autism Speaks - Autism science and advocacy organization
- http://www.autismspeaks.org/
Discussion Questions
- How can neuroimaging inform our understanding of the causes of autism?
- What are the ways in which neuroimaging, including fMRI and ERP, may benefit efforts to diagnosis and treat autism?
- How can an understanding of the social brain help us to understand ASD?
- What are the core symptoms of ASD, and why is the social brain of particular interest?
- What are some of the components of the social brain, and what functions do they serve?
Vocabulary
- Endophenotypes
- A characteristic that reflects a genetic liability for disease and a more basic component of a complex clinical presentation. Endophenotypes are less developmentally malleable than overt behavior.
- Measures the firing of groups of neurons in the cortex. As a person views or listens to specific types of information, neuronal activity creates small electrical currents that can be recorded from non-invasive sensors placed on the scalp. ERP provides excellent information about the timing of processing, clarifying brain activity at the millisecond pace at which it unfolds.
- Functional magnetic resonance imaging (fMRI)
- Entails the use of powerful magnets to measure the levels of oxygen within the brain that vary with changes in neural activity. That is, as the neurons in specific brain regions “work harder” when performing a specific task, they require more oxygen. By having people listen to or view social percepts in an MRI scanner, fMRI specifies the brain regions that evidence a relative increase in blood flow. In this way, fMRI provides excellent spatial information, pinpointing with millimeter accuracy, the brain regions most critical for different social processes.
- The set of neuroanatomical structures that allows us to understand the actions and intentions of other people.
References
Allison, T., Puce, A., & McCarthy, G. (2000). Social perception from visual cues: Role of the STS region. Trends in Cognitive Science, 4(7), 267–278.
Bentin, S., Allison, T., Puce, A., Perez, E., et al. (1996). Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience, 8(6), 551–565.
Brothers, L. (1990). The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience, 1, 27–51.
Dawson, G., Meltzoff, A. N., Osterling, J., Rinaldi, J., & Brown, E. (1998). Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism & Developmental Disorders, 28(6), 479–485.
Dawson, G., Webb, S. J., & McPartland, J. (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, 27(3), 403–424.
Elsabbagh, M., Mercure, E., Hudry, K., Chandler, S., Pasco, G., Charman, T., et al. (2012). Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology, 22(4), 338–342.
Geschwind, D. H., & Levitt, P. (2007). Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology, 17(1), 103–111.
Goren, C. C., Sarty, M., & Wu, P. Y. (1975). Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics, 56(4), 544–549.
Gottesman I. I., & Shields, J. (1973) Genetic theorizing and schizophrenia. British Journal of Psychiatry, 122, 15–30.
Hobson, R. (1986). The autistic child’s appraisal of expressions of emotion. Journal of Child Psychology and Psychiatry, 27(3), 321–342.
Johnson, M. H. (2006). Biological motion: A perceptual life detector? Current Biology, 16(10), R376–377.
Kaiser, M. D., Hudac, C. M., Shultz, S., Lee, S. M., Cheung, C., Berken, A. M., et al. (2010). Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America, 107(49), 21223–21228.
Kanner, L. (1943). Autistic disturbances of affective contact. Nervous Child, 2, 217–250.
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., & Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459(7244), 257–261.
Maestro, S., Muratori, F., Cavallaro, M. C., Pei, F., Stern, D., Golse, B., et al. (2002). Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 41(10), 1239–1245.
McCleery, J. P., Akshoomoff, N., Dobkins, K. R., & Carver, L. J. (2009). Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry, 66(10), 950–957.
McPartland, J. C., Dawson, G., Webb, S. J., Panagiotides, H., & Carver, L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 45(7), 1235–1245.
McPartland, J. C., Webb, S. J., Keehn, B., & Dawson, G. (2011). Patterns of visual attention to faces and objects in autism spectrum disorder. Journal of Autism and Develop Disorders, 41(2), 148–157.
Minshew, N. J., & Williams, D. L. (2007). The new neurobiology of autism: Cortex, connectivity, and neuronal organization. Archives of Neurology, 64(7), 945–950.
Osterling, J., & Dawson, G. (1994). Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders, 24, 247-257.
Osterling, J. A., Dawson, G., & Munson, J. A. (2002). Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development & Psychopathology, 14(2), 239–251.
Ozonoff, S., Iosif, A. M., Baguio, F., Cook, I. C., Hill, M. M., Hutman, T., et al. (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49(3), 256–266.
Pelphrey, K. A., Sasson, N. J., Reznick, J. S., Paul, G., Goldman, B. D., & Piven, J. (2002). Visual scanning of faces in autism. Journal of Autism & Developmental Disorders, 32(4), 249–261.
Schultz, R. T., Gauthier, I., Klin, A., Fulbright, R. K., Anderson, A. W., Volkmar, F., et al. (2000). Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry, 57(4), 331–340.
Simion, F., Regolin, L., & Bulf, H. (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences, 105(2), 809–813.
de Haan, M., Johnson, M. H., & Halit, H. (2003). Development of face-sensitive event-related potentials during infancy: A review. International Journal of Psychophysiology, 51(1), 45–58.
Attributions
Adapted by Kenneth A. Koenigshofer, PhD, from Pelphrey, K. A. (2021). Autism: insights from the study of the social brain. In R. Biswas-Diener & E. Diener (Eds), Noba textbook series: Psychology. Champaign, IL: DEF publishers. Retrieved from http://noba.to/yqdepwgt
Authors
- Dr. Kevin Pelphrey is the Harris Professor in the Yale Child Study Center and director of the Center for Translational Developmental Neuroscience and Center for Excellence in Autism Research and Treatment. His research focuses on the application of cognitive neuroscience and genetics to understanding the systems biology of neurodevelopmental disorders.
Creative Commons License



 Autism: Insights from the Study of the Social Brain by Kevin A. Pelphrey is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.
Autism: Insights from the Study of the Social Brain by Kevin A. Pelphrey is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. Permissions beyond the scope of this license may be available in our Licensing Agreement.


